Japan Asthma and COPD Therapeutics Market Analysis
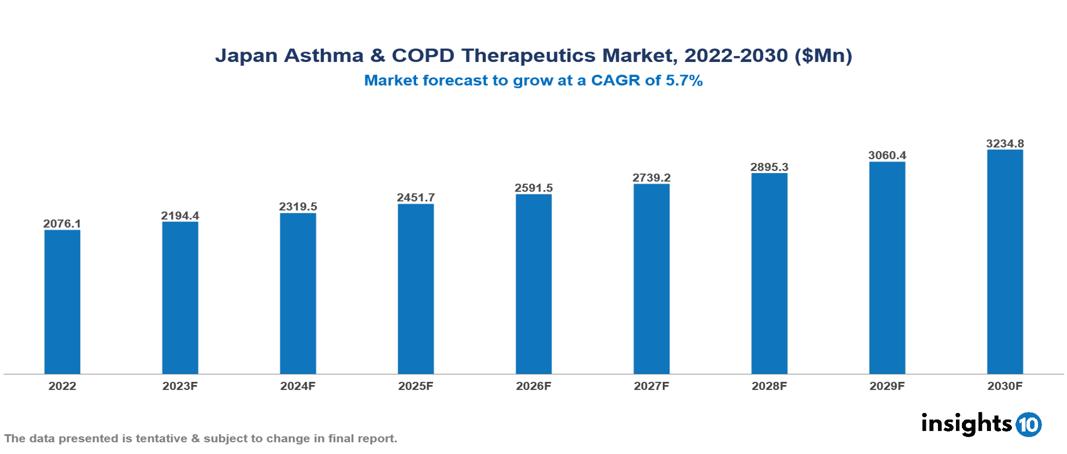
The Japan Asthma and COPD Therapeutics Market was valued at US $2.076 Bn in 2022, and is predicted to grow at (CAGR) of 5.70% from 2023 to 2030, to US $3.235 Bn by 2030. The key drivers of this industry include the surge in the prevalence of COPD and Asthma, supportive government initiatives, advancements in technology, and others. The industry is primarily dominated by players such as AstraZeneca, Novartis, Daiichi Sankyo, Chugai, Sumitomo, and Mochida, among others.
Buy Now

Japan Asthma and COPD Therapeutics Market Analysis: Executive Summary
The Japan Asthma and COPD Therapeutics Market is at around US $2.076 Bn in 2022 and is projected to reach US $3.235 Bn in 2030, exhibiting a CAGR of 5.70% during the forecast period.
Asthma and chronic obstructive pulmonary disease (COPD) are long-term respiratory conditions that compromise the airways and cause difficulty breathing. Asthma causes inflammation and constriction of the airways, frequently caused by irritants, allergens, or physical exercise. Symptoms include wheezing, shortness of breath, chest tightness, and coughing, with risk factors like respiratory infections and a family history of asthma. COPD, on the contrary, consists of emphysema and chronic bronchitis, both of which cause airway blockage. Smoking is a major risk factor for COPD, with symptoms including weariness, increased mucus production, and a persistent cough. Both conditions are commonly treated with bronchodilators to relieve airway constriction and anti-inflammatory medicines to reduce inflammation. GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim are widely recognized pharmaceutical companies that offer treatments for COPD and asthma.
COPD poses a considerable burden in Japan, with an estimated prevalence of around 10%, while asthma is prevalent at approximately 7%. These prevalence estimates are directly associated with risk factors, such as smoking, environmental pollution, and other contributing factors. The market is being driven by factors such as the rise in prevalence of asthma and COPD, supportive policies, technological advancements in the therapeutics industry, and others. However, high treatment costs like innovative biologics, pricing constraints, and competition from traditional medicines are a few factors that limit the market's potential.
Market Dynamics
Market Growth Drivers
Surge in prevalence: The prevalence of COPD is estimated to be around 10% in adults aged 40 years and older and can go as high as 30–40% in the smoking population of Japan. Subsequently, the prevalence of asthma is around 7%, accounting for roughly 3 million of the Japanese population. This can be attributed to factors such as smoking, increasing urbanization, and environmental pollution. As the population ages rapidly, there is an increasing need for the management of chronic conditions such as COPD and asthma, which drives the market for growth.
Technological advancements: The ongoing advancement of novel drugs exhibiting enhanced effectiveness and targeted therapies, such as biologics and combination treatments, is propelling market growth. The expansion of the market is also influenced by the introduction of modern inhaler technologies characterized by improved targeting and user-friendly features.
Government initiatives: Initiatives undertaken by the government to detect, prevent, and control respiratory diseases early on have the potential to boost market growth. Additionally, market accessibility and affordability are improved through favourable reimbursement policies for innovative therapies and respiratory devices in Japan. Japan is also a member of associations such as the Global Initiative for Asthma (GINA), which further attracts new pharmaceutical entrants to the Japanese markets.
Market Restraints
Pricing constraints: The Japanese government enforces stringent price controls and policies for drug reimbursement, compelling manufacturers to provide products at lower prices. This practice may constrain the acceptance of innovative and costly therapies, even when they demonstrate superior efficacy.
Increased competition: In Japan, there is widespread use of generic drugs, known for their affordability but potentially lacking the effectiveness of more recent branded alternatives. This situation disincentivizes and limits the accessibility of innovative therapeutics in the market.
High costs of treatment: Japan's healthcare system, characterized by multiple tiers and the involvement of various parties, results in a complex drug pricing framework. This complexity can impede patient access, as it may lead to elevated co-payments or restricted coverage for newer and more costly medications, resulting in treatment non-compliance in many patients.
Notable updates
September 2022, In Japan, AstraZeneca's Tezspire (tezepelumab) has received approval for addressing bronchial asthma in patients experiencing severe or refractory conditions where asthma symptoms remain unmanageable despite the use of mid- or high-dose inhaled corticosteroids and other long-term maintenance therapies.
Healthcare Policies and Regulatory Landscape
Japan's healthcare policy and regulatory framework comprise several key authorities and agencies. The primary entity overseeing healthcare regulations and licensing is the Ministry of Health, Labour, and Welfare (MHLW). The MHLW establishes the foundational laws for national health policy, which local governments implement. Furthermore, it serves as a crucial regulatory body for the Medical and Medical Practitioners Law, responsible for licensing medical practitioners and regulating medical devices.
Securing a license for healthcare products in Japan necessitates compliance with regulations set by the MHLW and local governments. These entities are responsible for supervising healthcare services, including the approval and monitoring of medical and healthcare products. Companies seeking registration and marketing authorization for pharmaceuticals and medical devices must obtain approval from the MHRA. This involves the submission of technical and scientific data to validate the safety, quality, and effectiveness of the product.
Japan's healthcare policy and regulatory structure involve a variety of authorities and agencies, with the MHLW playing a central role in the regulation of healthcare products. The diverse opportunities presented by both the public and private healthcare sectors in the country are attractive for companies operating within the healthcare industry.
Competitive Landscape
Key Players
- Novartis
- GlaxoSmithKline
- Teva Pharmaceutical Industries
- AstraZeneca
- Chugai Pharmaceutical
- Daiichi Sankyo
- Mitsubishi Tanabe Pharma
- Mochida Pharmaceutical Holdings
- Sumitomo Dainippon Pharma
- Fujifilm Toyo Pharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Japan Asthma and COPD Therapeutics Market Segmentation
By Disease Type
- Asthma
- COPD
By Medication Class
- Combination drugs
- Short Acting Beta Agonists (SABA)
- Long Acting Beta Agonists (LABA)
- Leukotriene Antagonists (LTA)
- Anticholinergics
- Others
By Delivery Device
- Metered dose inhalers (MDI)
- Dry Powder inhalers (DPI)
- Nebulizers
By Route of Administration
- Inhaled
- Oral
- Others
By End User
- Asthma Patients
- COPD Patients
By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.