Italy Oncology Therapeutics Market Analysis
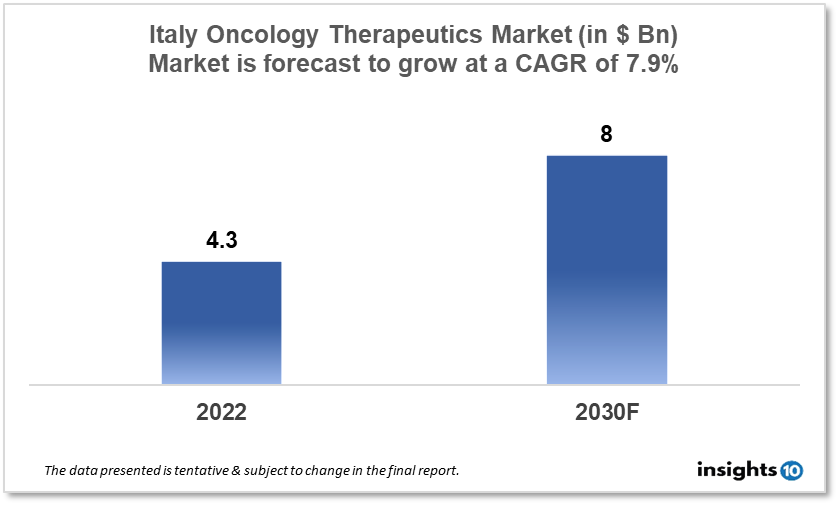
By 2030, it is anticipated that the Italy Oncology Therapeutics Market will reach a value of $8 Bn from $4.3 Bn in 2022, growing at a CAGR of 7.9% during 2022-2030. The Oncology Therapeutics Market in Italy is dominated by a few domestic pharmaceutical companies such as Dompe, MolMed, and Menarini. The Oncology Therapeutics Market in Italy is segmented into different types of cancer and different therapy type. The major risk factors associated with cancer are diet, alcohol, tobacco, air pollution, and physical inactivity. The demand for Italy Oncology Therapeutics is increasing on account of the rise in initiatives taken by the Government of the country.
Buy Now

Italy Oncology Therapeutics Market Analysis Summary
By 2030, it is anticipated that the Italy Oncology Therapeutics Market will reach a value of $8 Bn from $4.3 Bn in 2022, growing at a CAGR of 7.9% during 2022-2030.
Italy is a high-income, developed country located in Southern Europe comprising the boot-shaped Italian peninsula and several islands including Sicily and Sardinia. It is projected that approximately 377,000 new instances of malignant tumours will be diagnosed in Italy, with approximately 195,000 in males and approximately 182,000 in women. Every day in Italy, 1,030 people are diagnosed with cancer. Geographic comparisons reveal considerable North-South disparities in cancer prevalence in Italy. The average annual incidence rate in Central Italy and the South/Islands is lower.
Cancer is the main cause of mortality among people aged 30-69 in Italy. Cancer killed 180,085 individuals in Italy in 2017, accounting for 27.7 % of all deaths that year, with 100,123 men dying and 79,962 women dying. In Italy, alcohol consumption was responsible for 6.1 % of cancer deaths in men aged 15 and above. 7.2% of cancers are attributable to infections. Italy's government spends 9.6 % of its GDP on healthcare.
Market Dynamics
Market Growth Drivers
The 5-year survival rate in Italy is 54 % for males and 63 % for women, the highest in the European Union. The higher survival rate in women is since the most common cancer is breast cancer, which has a favourable prognosis when detected early. As a result, the national public healthcare system can give patients with effective and timely treatments. It is anticipated that more than 3.6 Mn Italians will have a cancer diagnosis by 2020, accounting for one in every 17 Italians, including 1.9 Mn women (6 % of the female population) and 1.7 Mn males (5.6 % of the male population). In Italy, tobacco smoking is the major cause of lung cancer, accounting for 85 to 90 % of cases. In Italy, the industrial sector is still highly regarded. These aspects could boost Italy Oncology Therapeutics Market.
Market Restraints
Despite the fact that Italy is a developed country with a strong healthcare system, funding for specialised research is restricted. This can make obtaining the necessary finances for research and development of new liver cancer treatments difficult. These factors may deter new entrants into the Italy Oncology Therapeutics Market.
Competitive Landscape
Key Players
- Menarini: Menarini is an Italian multinational pharmaceutical company that develops and produces a range of cancer therapeutics, including chemotherapy drugs and targeted therapies
- MolMed: MolMed is an Italian biotechnology company that specializes in the development of novel gene and cell therapies for the treatment of cancer. The company is currently developing several innovative CAR-T cell therapies for a range of cancer types
- Sigma-Tau: Sigma-Tau is an Italian pharmaceutical company that develops and produces a range of cancer therapeutics, including chemotherapy drugs and targeted therapies
- Dompe: Dompe is an Italian pharmaceutical company that focuses on the development and production of a range of cancer therapeutics, including chemotherapy drugs and immunotherapies
Notable Recent Deals
May 2022: Novartis has halted the manufacture of two cancer medications at plants in Italy and New Jersey owing to "possible quality concerns" discovered in the company's manufacturing procedures. As a result, the Swiss pharmaceutical company will temporarily suspend delivery of the two treatments, known as Lutathera and Pluvicto, while it tries to resolve the issue. Novartis stated that there is no indication of risk to patients treated with medication products previously manufactured at either of the locations and that treatment centres have been urged to monitor for any side effects.
Healthcare Policies and Reimbursement Scenarios
The Italian National Health Service manages public healthcare spending for oncological disease treatment. The Italian Medicines Agency (AIFA) is the regulatory authority in charge of the approval and regulation of therapeutic medicines, particularly cancer therapies, in Italy. The "Stati di Conoscenza" (State of Knowledge) system in Italy also evaluates and reimburses novel cancer treatments. This method permits patients suffering from life-threatening or serious conditions, such as cancer, to receive therapies that have not yet been licenced for use in Italy.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Oncology Therapeutics Segmentation
By Application (Revenue, USD Billion):
- Blood Cancer
- ?Colorectal Cancer
- Gastrointestinal Cancer
- Gynaecologic Cancer
- Breast Cancer
- Lung Cancer
- Prostate Cancer
- ?Others
By Drugs (Revenue, USD Billion):
- Revlimid
- Avastin
- Herceptin
- Rituxan
- Opdivo
- Gleevec
- Velcade
- Imbruvica
- Ibrance
- Zytiga
- Alimta
- Xtandi
- Tarceva
- Perjeta
- Temodar
- Others
By Therapy (Revenue, USD Billion):
- Chemotherapy
- Targeted Therapy
- Immunotherapy
- Hormonal Therapy
- Others
By Route of Administration (Revenue, USD Billion):
- Oral
- Parenteral
- Others
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- ?Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































