Italy Neurology Devices Market Analysis
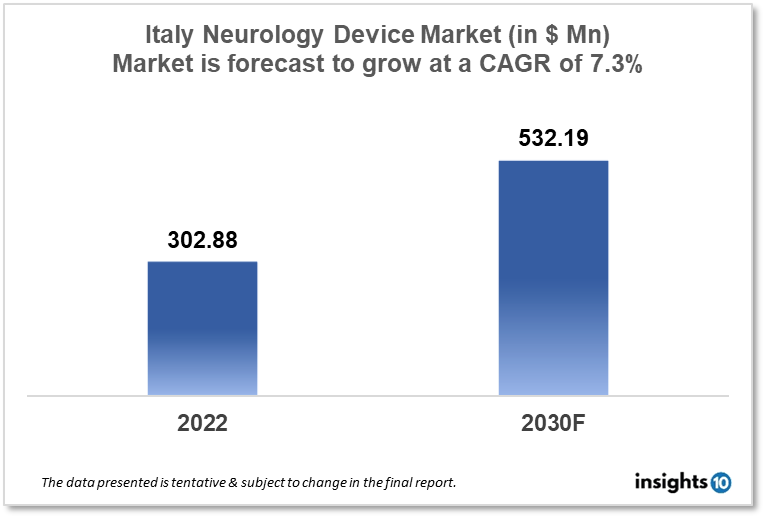
Italy's Neurology Device Market size is at around $302.88 Mn in 2022 and is projected to reach $532.19 Mn in 2030, exhibiting a CAGR of 7.3% during the forecast period. Italy has several world-renowned medical institutions, including the University of Bologna, the University of Milan, and the University of Turin, which have strong programs in neurology and neurosurgery. The market is dominated by Medtronic, NeuroPace, and St. Jude Medical. This report by Insights10 is segmented by product type like neurostimulation, interventional neurology, neurosurgery devices, and neuro-endoscopes, and by the end user.
Buy Now

Italy Neurology Device Market Executive Summary
Italy has a long history of excellence in the field of neurology, and there are many highly trained and skilled neurosurgeons practicing in the country. Italy has several world-renowned medical institutions, including the University of Bologna, the University of Milan, and the University of Turin, which have strong programs in neurology and neurosurgery. Italian neurosurgeons are known for their expertise in a range of procedures, including spinal surgery, neuro-oncology, and functional neurosurgery. In addition to their clinical work, Italian neurosurgeons are also actively engaged in research, working to advance the field and improve patient outcomes. Italy is home to a thriving community of neurosurgeons who are dedicated to providing high-quality care to their patients.
Neurology devices play an important role in the treatment of neurological conditions in Italy. Italy's Neurology Device Market size is at around $302.88 Mn in 2022 and is projected to reach $532.19 Mn in 2030, exhibiting a CAGR of 7.3% during the forecast period. These devices can be used to diagnose, monitor, and treat a wide range of conditions, including brain and spinal injuries, neurodegenerative diseases, movement disorders, and neuropsychiatric conditions. For example, neurostimulation devices such as deep brain stimulation (DBS) and transcranial magnetic stimulation (TMS) can be used to modify brain activity and alleviate symptoms of conditions such as Parkinson's disease, depression, and chronic pain. Interventional neuro devices, such as embolization coils and stents, can be used to treat conditions such as brain aneurysms, brain arteriovenous malformations (AVMs), and stroke. By using these technologies, doctors and surgeons in Italy can provide more targeted and effective treatment for patients with neurological conditions, potentially leading to improved outcomes and better quality of life. Additionally, the use of these devices can help to reduce the need for more invasive procedures, such as open brain surgery, and minimize the risk of complications.
The field of neurology devices is growing in Italy, with a growing interest in using technology to diagnose and treat neurological conditions. There are numerous companies and research institutions working on the development and implementation of various types of devices, including brain-machine interfaces, implantable devices for deep brain stimulation, and non-invasive techniques for transcranial stimulation. There is also a focus on improving existing techniques and developing new ones to increase their effectiveness and reduce side effects. Some of the most commonly used neurostimulation techniques in Italy include deep brain stimulation (DBS) for the treatment of movement disorders such as Parkinson's disease and transcranial magnetic stimulation (TMS) for the treatment of depression and other neuropsychiatric conditions. Interventional neuro devices, such as embolization coils and stents, are also commonly used to treat conditions such as brain aneurysms and strokes. The use of these technologies continues to evolve and change over time, as new treatments and devices become available and as the understanding of their effects in Post Market Survey (PMS) also helps improve feedback systems on the effectiveness and efficacy of the devices and their further development.
Market Dynamics
Market Growth Drivers
The need for effective treatments for neurological conditions can drive demand for neurology devices, as patients and their families seek out the best possible care. Positive clinical outcomes and positive patient experiences with neurology devices can increase demand and promote sales. Recommendations from healthcare providers, including neurosurgeons and neurologists, can help to increase the visibility and credibility of neurology devices, leading to increased demand and sales. Favorable reimbursement policies, such as those that provide coverage for neurology devices, can increase their availability and affordability, and promote sales. Technological advancements in neurology devices, such as the development of new and improved devices, can drive demand and increase sales. Effective marketing and promotion strategies, such as awareness campaigns and educational programs, can increase awareness of neurology devices and promote sales. Economic stability, and growth in consumer spending, can increase demand for healthcare products, including neurology devices.
Market Restraints
The level of reimbursement provided by the government and private insurance companies can affect the availability and affordability of these devices, which can in turn impact their sales. The presence of alternative treatments, such as drugs or other devices, can impact the demand for neurology devices. The level of competition among companies selling neurology devices can also affect sales. The regulatory framework for the approval and marketing of neurology devices can impact their availability and sales. Economic conditions, such as changes in the job market, consumer spending, and the overall state of the economy can impact the demand for these devices. The level of healthcare spending in Italy, and the allocation of resources for neurological treatments, can also impact the sales of neurology devices. The level of investment in research and development of new neurology devices can also impact their sales, as the availability of new and improved devices can drive demand.
Competitive Landscape
Key Players
- Medtronic
- Boston Scientific
- St. Jude Medical
- Neuromod
- NeuroPace
Healthcare Policies and Regulatory Landscape
In Italy, the regulatory body responsible for overseeing the approval and marketing of medical devices, including neurology devices, is the Italian Medicines Agency (AIFA). AIFA is responsible for evaluating the safety, efficacy, and quality of medical devices before they are placed on the market, and for monitoring their use once they are available to patients. AIFA also works to ensure that medical devices are used in a manner that is safe for patients and in accordance with established standards and regulations. AIFA plays an important role in ensuring the safety and effectiveness of neurology devices in Italy, and in promoting the development and availability of innovative and effective treatments for neurological conditions.
Reimbursement Scenario
Reimbursement policies play an important role in determining the availability and affordability of medical devices, including neurology devices. In Italy, the National Health Service (Servizio Sanitario Nazionale or SSN) provides coverage for a wide range of medical treatments, including many types of neurology devices. The level of reimbursement provided by the SSN for these devices can vary depending on the type of device and the specific indication for which it is used. In some cases, private insurance companies may also provide coverage for neurology devices. The level of coverage provided by these companies can vary and may depend on the specific policies and agreements in place. The reimbursement policies in Italy can play a significant role in determining the demand and availability of neurology devices, and companies must take these policies into consideration when developing and marketing their products.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Italy Neurology Device Market Segmentation
The Neurology Device Market is segmented as mentioned below:
By Product Type (Revenue, USD Billion):
- Neurostimulation
- Spinal Cord Stimulation Devices
- Deep Brain Stimulation Devices
- Sacral Nerve Stimulation
- Vagus Nerve Stimulation
- Gastric Electric Stimulation
- Interventional Neurology
- Aneurysm Coiling & Embolization
- Embolic Coils
- Flow Diversion Devices
- Liquid Embolic Agents
- Cerebral Balloon Angioplasty & Stenting
- Carotid Artery Stents
- Filter Devices
- Balloon Occlusion Devices
- Neurothrombectomy
- Clot Retriever
- Suction Aspiration Devices
- Snares
- CSF Management
- CSF Shunts
- CSF Drainage
- Neurosurgery Devices
- Ultrasonic Aspirators
- Stereotactic Systems
- Neuroendoscopes
- Aneurysm Clips
By End User (Revenue, USD Billion):
- Hospitals and Clinics
- Specialty Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































