Indonesia Neurology Devices Market Analysis
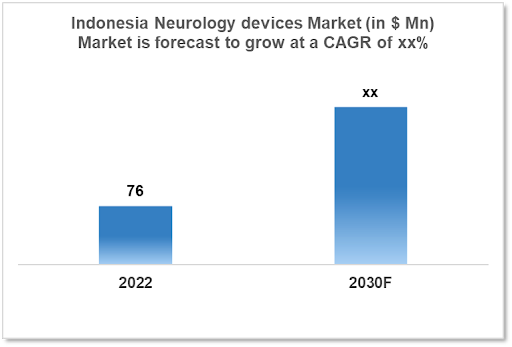
The Indonesia Neurology Devices Market size was valued at $76 Mn in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of xx% from 2022 to 2030 and will reach $xx Mn in 2030. The rising incidence of neurological illnesses in Indonesia is also a major factor in the demand for neurology devices The market is segmented by product type and by end user. Some key players in this market include Medtronic, Johnson and Johnson, Abbott Laboratories, Natus Medical, Elekta, and Integra LifeSciences.
Buy Now

Indonesia Neurology Devices Market Executive Analysis
The Indonesia Neurology Devices Market is at around $76 Mn in 2022 and is projected to reach $xx Mn in 2030, exhibiting a CAGR of xx% during the forecast period. Between 2022 and 2028, there was a projected steady rise in Indonesia's healthcare spending of $30.18 Bn. In 2028, spending is anticipated to be $69.3 Bn. Indonesia is regarded as a middle-income nation and is considered to be freshly industrialized. It ranks seventh in terms of GDP and is the 17th largest economy in the world by nominal GDP. Indonesia's Internet economy, which is projected to be worth $40 billion in 2019, is anticipated to reach $130 billion by 2025.
In Indonesia, neurological disorders are relatively common. The most common conditions include multiple sclerosis, Parkinson's disease, epilepsy, stroke, and Parkinson's disease. Numerous people in Indonesia are also affected by other ailments like migraine, neuropathic pain, and neuromuscular disorders. Depending on a number of variables, including age, gender, ethnicity, and geographic location within the nation, the precise prevalence and incidence rates of certain illnesses may change. The prevalence and incidence rates of neurological disorders in Indonesia may also be impacted by access to healthcare resources and services. Although deep brain stimulation devices assist in executing treatments by implanting a medical device that provides electrical impulses to particular target locations in the brain, interventional devices use radiology and catheters to diagnose the medical disorders present in the central nervous system. These tools are essential for the treatment of complicated illnesses. As a result, they are frequently used in the treatment of a variety of neurological illnesses, including major depressive disorder, Parkinson's disease, dystonia, chronic pain, and essential tremor.
Market Dynamics
Market Growth Drivers
One of the major factors boosting demand for neurology devices in Indonesia is the rising incidence of neurological disorders. Diabetes mellitus, obesity, high blood pressure, and hypertension are all associated with a higher occurrence of neurological diseases. Additionally, the number of such individuals in Indonesia is increasing significantly. The rising incidence of neurological illnesses in Indonesia is also a major factor in the demand for neurology devices. The adoption of neurology devices is being accelerated by the aging population as well as the increased rates of neurovascular disorders like stroke among the elderly, which is boosting the market growth. Additionally, the widespread need for less invasive operations and ongoing technological developments in neurology devices are both key growth-inducing drivers. Additional drivers of market expansion include rising healthcare costs, large investments made by international producers of medical devices, and extensive research and development (R&D) in the area of Neurotherapy.
Market Restraints
Neurology device makers may experience higher expenses and a longer approval procedure due to strict regulatory standards. The regulatory environment for neurology devices, particularly neurological devices, is heavily controlled in Indonesia, and following the rules can be challenging and time-consuming. This might make it harder for cutting-edge and innovative technologies to get started.
Competitive Landscape
Key Players
- Medtronic
- Abbott Laboratories
- Nihon Kohden
- Natus Medical
- Johnson and Johnson
- Elekta
- Integra LifeSciences
Healthcare Policies and Regulatory Landscape
The National Agency of Drug and Food Control (NA-DFC), a governmental body in charge of regulating the security, caliber, and effectiveness of medical goods, is in charge of overseeing the regulation of neurological devices in Indonesia. Medical device makers must adhere to the standards established by the National Agency of Drug and Food Control (NA-DFC) in order to access the market in Indonesia, where the regulatory environment for medical devices is continually changing. The regulatory framework and healthcare policies in Indonesia are geared towards enhancing the standard of care and ease of access to medical services, especially in the field of neurology.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Neurology Device Market Segmentation
The Neurology Device Market is segmented as mentioned below:
By Product Type (Revenue, USD Billion):
- Neurostimulation
- ?Spinal Cord Stimulation Devices
- Deep Brain Stimulation Devices
- Sacral Nerve Stimulation
- Vagus Nerve Stimulation
- Gastric Electric Stimulation
- Interventional Neurology
- Aneurysm Coiling & Embolization
- Embolic Coils
- Flow Diversion Devices
- Liquid Embolic Agents
- Cerebral Balloon Angioplasty & Stenting
- Carotid Artery Stents
- Filter Devices
- Balloon Occlusion Devices
- Neurothrombectomy
- Clot Retriever
- Suction Aspiration Devices
- Snares
- CSF Management
- CSF Shunts
- CSF Drainage
- Neurosurgery Devices
- Ultrasonic Aspirators
- Stereotactic Systems
- Neuroendoscopes
- Aneurysm Clips
By End User (Revenue, USD Billion):
- ??Hospitals and Clinics
- Specialty Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































