Indonesia Liver Cancer Therapeutics Market Analysis
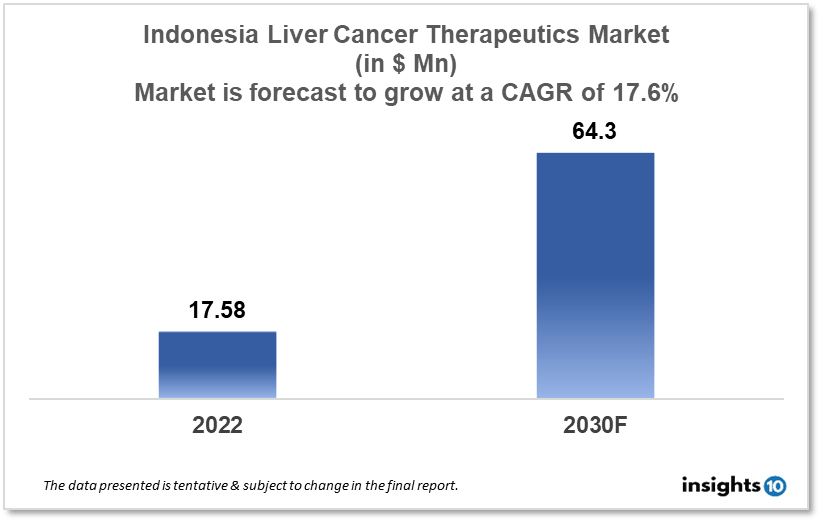
By 2030, it is anticipated that the Indonesia liver cancer therapeutics market will reach a value of $64.3 Mn from $17.58 Mn in 2022, growing at a CAGR of 17.6% during 2022-30. The liver cancer therapeutics market in Indonesia is dominated by a few domestic pharmaceutical companies such as Chugai Pharmaceutical, Taiho Pharmaceutical, and Eisai. The liver cancer therapeutics market in Indonesia is segmented into different types of cancer and different therapy type. Some of the major factors affecting this market are the growing prevalence of liver cancer and the high cost and side effects related to certain liver cancer therapies.
Buy Now

Indonesia Liver Cancer Therapeutics Analysis Summary
By 2030, it is anticipated that the Indonesia liver cancer therapeutics market will reach a value of $64.3 Mn from $17.58 Mn in 2022, growing at a CAGR of 17.6% during 2022-2030.
Indonesia is a lower middle-income, developing country located in Southeastern Asia between the Indonesian Ocean and the Pacific Ocean. According to the latest WHO data published in 2020 Liver Cancer Deaths in Indonesia reached 19,721 or 1.17% of total deaths. The age-adjusted Death Rate is 8.53 per 100,000 population ranks Indonesia 43rd in the world. As per IARC, the number of liver cancer cases due to alcohol consumption is 37% out of all gastrointestinal cancers.
In Indonesia, traditional medicine plays an essential role in the treatment of liver cancer. To control symptoms and enhance their quality of life, many patients in Indonesia use traditional medicines such as herbal remedies, acupuncture, and other traditional therapies. Soursop leaves, Java tea leaves, and Temu Kunci are some of the most often utilized herbal treatments. Indonesia's government spent 3.4 % of its GDP on healthcare in 2020.
Market Dynamics
Market Growth Drivers
There have been numerous advancements in liver cancer therapies in Indonesia in recent years. The availability of targeted medicines such as sorafenib and lenvatinib, which have been demonstrated to be effective in treating advanced HCC, has been one of the primary growth reasons. Furthermore, progress has been made in the development of immunotherapies for liver cancer, with medications like nivolumab and pembrolizumab showing promise in clinical trials. When doing business in Indonesia, low labor costs and a demographic dividend provide a competitive edge. Indonesia's Business Process Outsourcing (BPO) business is booming.
Market Restraints
Despite the government's efforts in healthcare, the amount of funding available for individual research programs remains restricted. This can make obtaining the necessary finances for research and development of new liver cancer treatments difficult. Indonesia is exposed to fluctuations in Chinese demand, as well as persistent corruption and a lack of transparency.
Competitive Landscape
Key Players
- Dexa Medica - Dexa Medica is one of the largest pharmaceutical companies in Indonesia. The company produces a range of generic and branded medicines, including oncology drugs. Dexa Medica produces several medicines for the treatment of liver cancer, including Sorafenib and Lenvatinib.
- Kalbe Farma - Kalbe Farma is another Indonesian pharmaceutical company that produces a range of medicines, including oncology drugs
- Tempo Scan Pacific - PT. Tempo Scan Pacific is an Indonesian-based pharmaceutical company that specializes in the development, manufacturing, and distribution of a range of medicines, including oncology drugs
- Prodia Widyahusada
- Kimia Farma
Healthcare Policies and Reimbursement Scenarios
In Indonesia, the regulation of liver cancer therapeutics is overseen by the National Agency of Drug and Food Control (BPOM), which is responsible for evaluating the safety, efficacy, and quality of all medicines, including those used for the treatment of liver cancer. Regarding reimbursement, Indonesia has a national health insurance program called the National Health Insurance (JKN), which provides coverage for a range of liver cancer therapeutics, including chemotherapy, targeted therapy, and immunotherapy.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Indonesia Liver Cancer Therapeutics Segmentation
By Type (Revenue, USD Billion):
- Hepatocellular Carcinoma
- Cholangio Carcinoma
- Hepatoblastoma
- Other Types
By Treatment (Revenue, USD Billion):
- Chemotherapy
- Immunotherapy
- Targeted Therapy
- Radiation Therapy
- Ablation Therapy
- Embolization Therapy
By Equipment (Revenue, USD Billion):
- Computed Radiography
- MRI
- Sonography
- Others
By Route of Administration (Revenue, USD Billion):
- Oral
- Intravenous
By End User (Revenue, USD Billion):
- Hospitals
- Clinics
- Ambulatory Surgical Centers
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































