Indonesia Hepatitis A Therapeutics Market Analysis
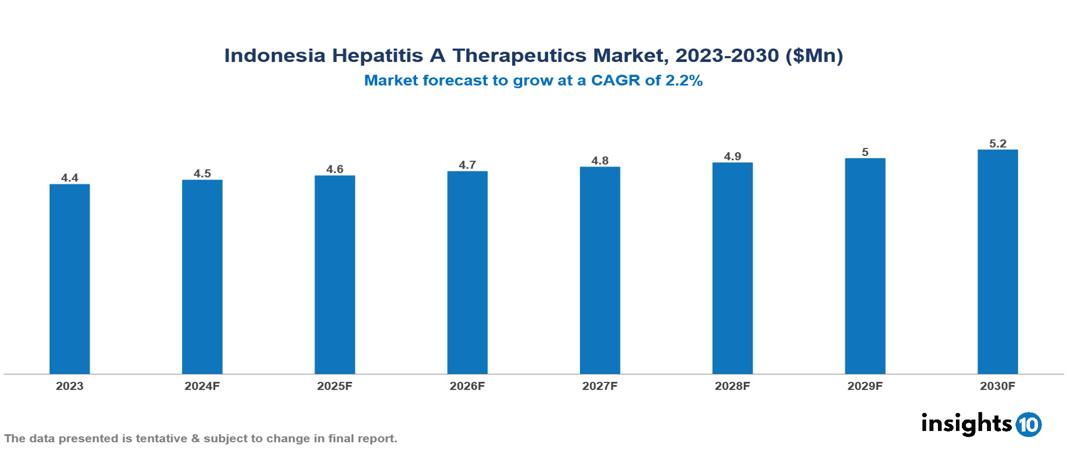
The Indonesia Hepatitis A Therapeutics Market was valued at $4.42 Mn in 2023 and is predicted to grow at a CAGR of 2.2% from 2023 to 2030 to $5.15 Mn by 2030. This growth is primarily driven by rising vaccination rates, the cost-effectiveness of treatments, and the growing middle-class population. Prominent companies in this market include Combiphar and F. Hoffmann-La Roche Ltd., among others
Buy Now

Indonesia Hepatitis A Therapeutics Market Executive Summary
The Indonesia Hepatitis A Therapeutics Market was valued at $4.42 Mn in 2023 and is predicted to grow at a CAGR of 2.2% from 2023 to 2030 to $5.15 Mn by 2030.
Hepatitis A is a viral liver infection that spreads through close contact with an infected person or by consuming contaminated food or water. Symptoms range from mild to severe and include fatigue, fever, loss of appetite, nausea, dark urine, and jaundice. Treatment generally involves rest, avoiding alcohol, and taking specific medications. Most people recover within a few weeks, but in some cases, the infection can last for months and lead to severe complications like liver failure. The Hepatitis A vaccine is highly effective and recommended for children, travelers to high-risk regions, and individuals with certain risk factors.
The cost-effectiveness of Hepatitis A vaccination in Indonesia is driven by its affordability and effectiveness, with a single-dose vaccine yielding an ICER of $4933 per quality adjusted life years (QALY) at a vaccine price of $3.21 per dose. This factor drives the market for Hepatitis A therapeutics. Rising vaccination rates, cost-effective treatments, and a growing middle class drive the market. On the contrary, uneven workforce distribution, skilled healthcare workforce shortage, lack of awareness and education restrain the market.
Market Dynamics
Market Growth Drivers
Rising Vaccination Rates: Efforts to increase awareness about Hepatitis A and promote vaccination, particularly among high-risk groups such as children and travelers, are growing in Indonesia. These efforts significantly contribute to the prevention and control of the disease and drive the market demand for therapeutics.
Cost-effectiveness of Therapeutics: The cost-effectiveness of Hepatitis A therapeutics is a key market driver in Indonesia. Studies have shown that Hepatitis A vaccination is both valuable and cost-effective. With a vaccine priced at $3.21 per dose, implementing a single-dose Hepatitis A vaccine yields an incremental cost-effectiveness ratio (ICER) of $4933 per QALY (Quality-Adjusted Life Year). This affordability and effectiveness increase demand for Hepatitis A treatments in the country.
Growing Middle Class: The expanding middle class in Indonesia, with 52 Mn economically secure individuals, drives demand for Hepatitis A treatments. Their increasing consumption, significant out-of-pocket payments for private healthcare, and the growth of private hospitals (a 24% increase from 2014 to 2018) cater to their healthcare needs. The Coordination of Benefits (COB) scheme further encourages middle-class Indonesians to seek higher-value medical services, boosting the Hepatitis A therapeutics market.
Market Restraints
Uneven Workforce Distribution: The unequal distribution of the pharmacy workforce in Indonesia, with a higher concentration in the capital city and shortages in rural and remote areas, affects accessibility to pharmaceutical expertise and primary healthcare support. This imbalance leads to shortages in pharmaceutical care services, particularly in underserved regions, acting as a market restraint for Hepatitis A therapeutics in Indonesia.
Skilled Healthcare Workforce Shortage: Indonesia's healthcare system is challenged by a shortage of skilled health personnel, with a density of only 15.54 per 10,000 population. The lack of healthcare professionals, especially liver disease specialists, limits expertise in diagnosing and treating Hepatitis A, restraining the growth of the Hepatitis A therapeutics market in the country.
Lack of Awareness and Education: Despite the high endemicity of Hepatitis A in Indonesia, there is limited awareness and education about the disease. Enhanced knowledge dissemination is needed to improve understanding. The scarcity of genetic information on HAV and the WHO's emphasis on sanitation, food safety, and immunization highlights the importance of awareness in preventing Hepatitis A transmission. This lack of awareness hinders understanding of the disease, its transmission, and prevention methods, negatively impacting treatment adoption and market growth.
Regulatory Landscape and Reimbursement Scenario
The reimbursement scenario for Hepatitis A therapeutics in Indonesia is complex due to a developing healthcare system and ongoing reforms. The National Health Insurance System (JKN) covers many essential medications, but coverage for specific Hepatitis A treatments might vary. Pharmaceutical companies negotiate with the Ministry of Health to get their medicines included on the JKN formulary, considering factors like cost, clinical evidence, and patient population targeted.
Regulatory bodies like the National Agency of Drug and Food Control (NADFC) ensure pharmaceuticals' safety, quality, and efficacy in Indonesia, overseeing the drug registration application review and granting drug approvals through marketing authorizations. Patients typically do not directly manage reimbursement, and out-of-pocket costs might vary depending on the specific medication, JKN coverage level, and healthcare provider. Healthcare providers prescribe Hepatitis A therapeutics based on best practices and patient needs, staying informed about updates to JKN formularies and reimbursement policies.
Competitive Landscape
Key Players
Here are some of the major key players in the Hepatitis A Therapeutics Market:
- Combiphar
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- Zydus Cadilla
- Sanofi
- GlaxoSmithKline (GSK)
- Takeda
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Indonesia Hepatitis A Therapeutics Market Segmentation
By Distribution Channel
- Hospital-based pharmacies
- Retail pharmacies
- Online pharmacies
By Route of Administration
- Oral Medications
- Intravenous Therapy
By Healthcare Setting
- Outpatient Care
- Inpatient Care
By Age
- Children
- Adults
- Senior Citizens
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































