Indonesia Conjunctivitis Therapeutics Market Analysis
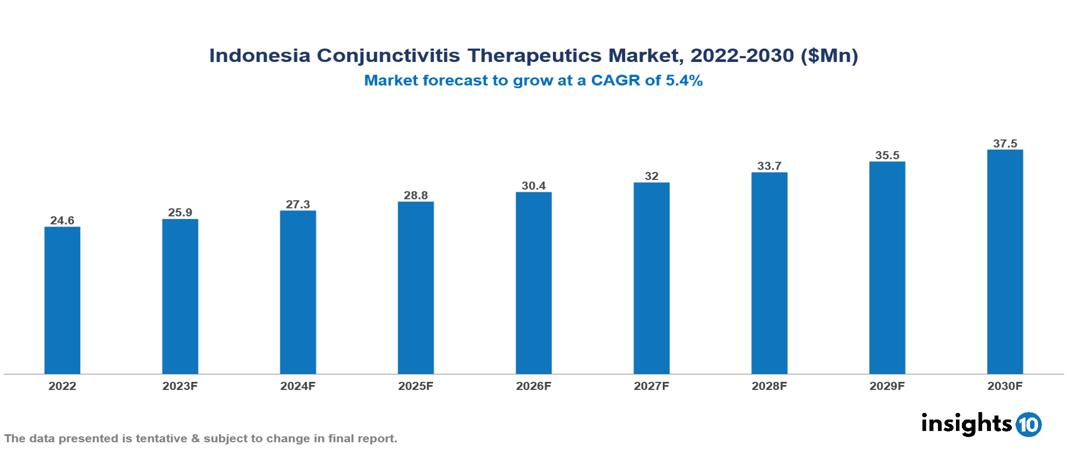
The Indonesia Conjunctivitis Therapeutics Market was valued at $25 Mn in 2022 and is predicted to grow at a CAGR of 5.4% from 2023 to 2030, to $37 Mn by 2030. The primary factors propelling this industry forward include the increasing prevalence of conjunctivitis, technological advancements, increasing government focus on preventative care, and others. The industry is primarily dominated by players such as Allergan, Bausch & Lomb, Alcon, Roche, Novartis, Santen, and Johnson & Johnson among others.
Buy Now

Indonesia Conjunctivitis Therapeutics Market Analysis Executive Summary
The Indonesia Conjunctivitis Therapeutics Market is at around $25 Mn in 2022 and is projected to reach $37 Mn in 2030, exhibiting a CAGR of 5.4% during the forecast period.
Conjunctivitis, commonly called pink eye, is the inflammation of the thin membrane that covers the eyelids and the whites of the eyes. Various factors , including viruses, bacteria, allergies, and irritants, can trigger this condition. Viral conjunctivitis, often initiated by the adenovirus responsible for the common cold, is highly contagious and typically initiates in one eye before spreading to the other. Symptoms vary depending on the underlying cause but generally encompass redness in the whites of the eyes, a gritty sensation, excessive tearing, itching, swollen eyelids, and discharge. Although the condition often resolves on its own within a few weeks, seeking medical attention becomes crucial if severe pain, changes in vision, light sensitivity, persistent discharge, or worsening symptoms arise. Treatment is contingent on the specific cause of conjunctivitis. Symptom management with pain relievers and cool compresses is common for viral cases, antibiotic eye drops are prescribed for bacterial cases, and antihistamine eye drops or oral medications are recommended for allergic cases. Companies such as Alcon, Allergan, Bausch & Lomb, Pfizer, and Santen Pharmaceutical produce these treatments.
Indonesia is experiencing an increasing burden of conjunctivitis which affects more than 73% of the total population in the country. Market growth is driven by factors such as the increase in the prevalence of conjunctivitis, technological advancements, increasing government focus on preventative care, and others. However, conditions such as poor healthcare system, affordability challenges, and lack of awareness in the population limit the growth and potential of the market.
Market Dynamics
Market Growth Drivers
The upward trend in the prevalence of conjunctivitis: The prevalence of conjunctivitis is estimated to be as high as 73% of the Indonesian population. The increasing urbanization and industrialization in Indonesia result in elevated levels of air pollution, a recognized factor that heightens the risk of conjunctivitis. The rise in the usage of digital devices and contact lenses also contributes to the transmission of conjunctivitis. This translates into a pool of patients requiring effective treatments.
Technological advancements: Pharmaceutical firms are working on the creation of advanced treatments for conjunctivitis that are not only more efficient but also more convenient, such as topical formulations and targeted therapies. The incorporation of digital technologies, such as AI-powered diagnostics and remote patient monitoring, has the potential to enhance both diagnosis and treatment, potentially expanding the market size.
Government focuses on preventative care: Government and healthcare organizations are progressively advocating awareness initiatives regarding eye hygiene practices and the significance of eye protection to prevent conjunctivitis. Programs focusing on enhancing the eye health of children such as the “Ch(eye)ld Health Program” including early identification and treatment of childhood conjunctivitis, have the potential to increase treatment seeking in the country.
Untapped market potential: At present, only a limited number of Indonesians affected by conjunctivitis actively pursue treatment, indicating a substantial untapped market opportunity for pharmaceutical companies.
Market Restraints
Poor healthcare system: The unequal distribution of healthcare facilities, especially in rural areas, hampers the accessibility of diagnosis and treatment for conjunctivitis. The absence of proficient healthcare professionals, particularly ophthalmologists, results in misdiagnosis and delayed treatment. Additionally, insufficient infrastructure, including restricted access to clean water and sanitation, can contribute to the transmission of conjunctivitis.
Affordability challenges: The high prices of branded therapeutic medications, especially recent innovations, can render them inaccessible to a substantial segment of the population. In addition, insufficient health insurance coverage for eye care and conjunctivitis treatment can impose additional financial strain on patients. Government and private insurer reimbursement policies may not fully cover the expenses associated with specific treatments.
Lack of awareness: Insufficient public knowledge regarding the cause, signs, and available treatments for conjunctivitis results in delayed identification and inappropriate treatment. Resorting to self-medication using over-the-counter medications or traditional remedies can exacerbate the condition and give rise to complications restricting the growth of the population.
Healthcare Policies and Regulatory Landscape
The main regulatory body overseeing drugs and pharmaceuticals in Indonesia is the National Agency of Drug and Food Control (Badan Pengawas Obat dan Makanan or BPOM). BPOM is responsible for ensuring the safety, quality, and efficacy of pharmaceuticals, as well as regulating their distribution and marketing in the country. The agency establishes and enforces standards for the registration, manufacturing, and marketing of drugs, and it plays a crucial role in safeguarding public health.
To obtain licensure for drugs and pharmaceuticals in Indonesia, manufacturers must undergo a rigorous regulatory process outlined by BPOM. This typically involves submitting comprehensive documentation on the product, including data on its safety, efficacy, and quality. The agency conducts thorough reviews and inspections to assess compliance with regulatory standards. Once approved, the product is granted marketing authorization, allowing it to be sold and distributed in the Indonesian market.
The regulatory environment for new entrants can be challenging due to the stringent requirements set by BPOM, but adherence to these regulations is essential to ensure the safety and efficacy of pharmaceuticals in the Indonesian market. New entrants have to invest time and resources to navigate the regulatory landscape and meet the necessary criteria for product approval.
Competitive Landscape
Key Players
- Alcon
- Allergan
- Bausch & Lomb
- Santen Pharmaceutical
- Novartis
- Pfizer
- Merck & Co
- Johnson & Johnson
- Roche
- Abbott Pharmaceuticals
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Indonesia Conjunctivitis Therapeutics Market Segmentation
By Drug Class
- Antibiotics
- Antiviral
- Antiallergic
- Others
By Treatment
- Mast Cell Stabilizers
- Decongestant
- Immunotherapy
- Antihistamines
- Non-steroidal anti-inflammatory drugs
- Olopatadine
- Epinastine
- Others
By Disease Type
- Bacterial
- Chemical
- Viral
- Allergic
By Formulation
- Ointment
- Drops
- Drugs
By End Users
- Hospitals and clinics
- Online Pharmacies
- Retail Pharmacies
- Drug Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.