Indonesia Congestive Heart Failure Therapeutics Market Analysis
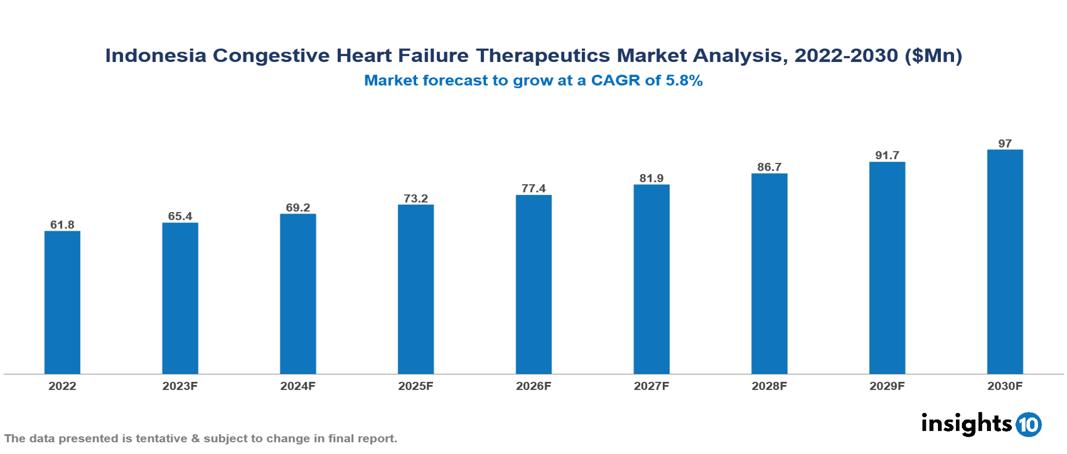
The Indonesia Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $62 Mn in 2022 to $97 Mn by 2030, with a CAGR of 5.8% during the forecast period of 2022-2030. The growth of the Indonesia Congestive Heart Failure Therapeutics Market is driven by the increasing prevalence of cardiovascular conditions among an aging population, government initiatives enhancing accessibility and affordability, and the potential for healthcare infrastructure improvements, particularly in rural areas. The Indonesia Congestive Heart Failure Therapeutics Market encompasses various key players across different therapeutic segments, including Novartis, Sanofi, AstraZeneca, Bayer, Eli Lilly, Merck, Kalbe Pharma, Kimia Pharma, Pharos, Indopharma, etc., among various others.
Buy Now

Indonesia Congestive Heart Failure Therapeutics Market Analysis Executive Summary
The Indonesia Congestive Heart Failure Therapeutics Market is anticipated to experience a growth from $62 Mn in 2022 to $97 Mn by 2030, with a CAGR of 5.8% during the forecast period of 2022-2030.
Congestive heart failure (CHF) is a chronic disorder, characterized by the heart's inability to pump blood effectively, which results in insufficient oxygen delivery to the body's tissues. Heart conditions such as coronary artery disease, hypertension, or myocardial infarction frequently cause CHF. Additionally, several different factors can lead to congenital cardiac defects, high blood pressure, injury to the heart muscle, and valve problems. Lifestyle factors that may also be associated with CHF include smoking, obesity, and excessive alcohol intake. CHF can be classified into two categories: systolic, in which the heart has trouble contracting and pumping blood, and diastolic, in which the heart has trouble relaxing and filling with blood. To treat CHF, a multidisciplinary strategy is usually applied. For treating symptoms and enhancing heart health, doctors frequently prescribe anti-hypertensive drugs such as ACE inhibitors, beta-blockers, and diuretics. A heart-healthy diet, consistent exercise, and stress reduction are all important lifestyle changes. Advanced interventional options like surgical procedures such as heart valve replacement or repair may be considered in extreme circumstances.
The Southeast Asian region generally has a higher frequency of Heart Failure (HF) cases than Western countries with younger patients and more co-morbid conditions. The estimated prevalence of CHF in Indonesia is about 6.5%. Atrial fibrillation, diabetes mellitus, coronary artery disease, and hypertension are the most common comorbidities for those with CHF in the country.
The growth of the Indonesia Congestive Heart Failure Therapeutics Market is driven by the increasing prevalence of cardiovascular conditions among an aging population, government initiatives enhancing accessibility and affordability, and the potential for healthcare infrastructure improvements, particularly in rural areas.
Among the most renowned global players, Novartis and Sanofi are known for their long-standing brands such as Diovan. Kalbe Farma is the leader in the domestic market thanks to its well-known brands, wide range of products, and efficient distribution channel. Kimia Farma has a large market share but does not have strong brands or many different types of products. Indofarma & Pharos provides the best accessibility and pricing because their primary focus is on generics unlike the state-owned companies’ strategy; however, they still lack brand image and a variety of products.
Market Dynamics
Market Growth Drivers
Prevalence of CVDs: The increasing incidence of heart failure in Indonesia is closely associated with the aging population in the nation, which is leading to an increasing burden of chronic illnesses. The need for efficient healthcare solutions is highlighted by the direct increase in demand for CHF drugs brought about by this demographic shift.
Governmental programs: The national health insurance program, BPJS Kesehatan, is one of the government's efforts to provide greater accessibility to necessary medications, particularly those that are critical for controlling CHF. Furthermore, pricing limitations and regulatory policies that encourage the use of generic drugs have a big impact on market dynamics, affecting availability and affordability.
Untapped Potential: Especially in terms of healthcare infrastructure, which faces limitations, particularly in rural areas. This situation presents a unique opportunity for companies to contribute to improving access and distribution networks for CHF medications, addressing gaps in healthcare delivery across the nation. As Indonesia continues to grapple with the evolving landscape of cardiovascular health, these factors collectively shape the dynamics of the CHF market in the country.
Market Restraints
Expensive Treatments: Affordability remains a pressing concern in Indonesia's healthcare landscape, despite commendable efforts by government initiatives like BPJS to enhance medication access. The substantial out-of-pocket expenses, particularly for branded cardiovascular drugs, pose a significant barrier and may affect patients' adherence to prescribed treatment plans, raising questions about the sustainability of long-term care.
Issue of Counterfeit Drugs: The persistent issue of counterfeit drugs continues to undermine patient safety and trust in legitimate pharmaceutical suppliers. Despite ongoing efforts to combat this problem, the prevalence of fake medications poses a threat to market stability and the overall integrity of the pharmaceutical sector, necessitating continuous vigilance and regulatory measures.
Shortage of Qualified Professionals: The brain drain, where skilled healthcare professionals migrate abroad, worsens the shortage of specialists in Indonesia. This lack of specialists affects the quality of care for CHF patients, highlighting the necessity for strategies to retain and attract talented medical professionals.
Healthcare Policies and Regulatory Landscape
The government plays a vital role in creating and implementing laws that govern the healthcare industry, covering topics such as infrastructure development, healthcare financing, and pharmaceutical regulatory systems. The National Agency of Drug and Food Control (BPOM), Indonesia's drug regulatory organization, is responsible for monitoring and controlling the pharmaceutical sector. BPOM guarantees that medications adhere to rigorous safety, efficacy, and quality standards before being released to the public. It establishes guidelines for pharmaceutical companies, performs comprehensive inspections, and enforces regulatory compliance according to healthcare policies. Additionally, BPOM collaborates with international regulatory bodies and public health agencies to maintain high-quality global standards.
Competitive Landscape
Key Players:
- Novartis
- Sanofi
- AstraZeneca
- Bayer
- Eli Lilly
- Merck
- Kalbe Pharma
- Kimia Pharma
- Pharos
- Indopharma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Indonesia Congestive Heart Failure Therapeutics Market Segmentation
By Stage of Heart Failure
- Acute Heart Failure
- Chronic Heart Failure
By Drug Class
- ACE Inhibitors
- Beta Blockers
- Angiotensin 2 Receptor Blockers
- Diuretics
- Aldosterone Antagonists
- Others
By Route of Administration
- Oral
- Parenteral
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.