Indonesia Blood Disorder Therapeutics Market
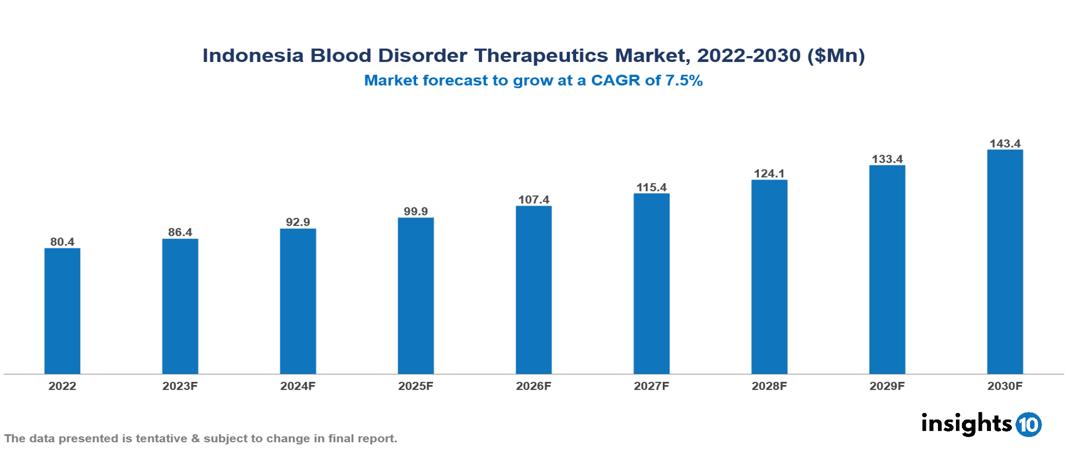
Indonesia Blood Disorder Therapeutics Market valued at $80 Mn in 2022, projected to reach $143 Mn by 2030 with a 7.5% CAGR. The market growth is fuelled due to certain factors such as the increasing incidence of blood disorders in Indonesia, driven by improved diagnostics and accessibility, heightened awareness, identification of genetic and environmental risk factors, and rising investments in healthcare. The Indonesia Blood Disorder Therapeutics Market encompasses various players across different segments, including Roche, Novartis, Pfizer, Bayer, BioMarin Pharmaceutical, Takeda, Kalbe Farma, Dexa Medica, Combiphar, Kimia Farma, etc, among various others.
Buy Now

Indonesia Blood Disorder Therapeutics Market Executive Summary
Indonesia Blood Disorder Therapeutics Market valued at $80 Mn in 2022, projected to reach $143 Mn by 2030 with a 7.5% CAGR.
Blood disorders include an extensive spectrum of illnesses that impair the regular operation of blood constituents such as plasma, red blood cells, and white blood cells. These illnesses frequently cause a decrease in the number of proteins, platelets, blood cells, or vital nutrients, which interferes with their ability to operate properly. Genetic mutations are the root cause of many blood illnesses, and many of them have a hereditary component. They might not be malignant or they might be. Blood problems can be treated in a variety of ways, from straightforward dietary changes to more involved medical procedures. Invasive therapies are required in some situations. To target and eradicate aberrant cells, for example, chemotherapy and radiation treatment may be used. Furthermore, novel strategies such as tailored drug therapy can improve chemotherapy outcomes or target particular aspects of cancer cells that traditional drugs may miss. Stem cell transplantation is also an option, particularly in cases when more direct intervention is required to treat certain blood-related problems.
Anemia, the most prevalent blood disorder, is a major health problem in Indonesia, with a frequency of 22.7% in women of reproductive age, 37.1% among pregnant women, and 30.0% to 46.6% among female workers. Additionally, Indonesia is a hub for hemoglobinopathies and is situated along the "Thalassemia Belt." About 3.0-10.1% and 2.6-11.0% of people, respectively, are affected by β- and β-thalassemia.
The market growth is fueled by certain factors such as the increasing incidence of blood disorders in Indonesia, driven by improved diagnostics and accessibility, heightened awareness, identification of genetic and environmental risk factors, and rising investments in healthcare.
Leading global pharma companies like Novartis and Roche, as well as emerging local firms like Kalbe Farma and Dexa Medica, are present in the Indonesia Blood Disease Treatment Market. Due to their extensive distribution networks and well-known brands, international businesses now have the highest market share, but domestic competitors are starting to gain traction since they are more reasonably priced and sensitive to local demands.
Market Dynamics
Market Growth Drivers
Growing Blood Disorder Incidence: Blood diseases such as thalassemia, hemophilia, and sickle cell anemia are becoming more common in Indonesia. This can be attributed to improved diagnostics, higher awareness, and increasing identification of risk factors like genetic predisposition and environmental changes. Additionally, the growing population adds to the number of people who might be impacted.
Increase in Investment in Healthcare: Driven by growing economic prosperity, the Indonesian government is allocating more funds for healthcare, including raising the national health insurance program's (JKN) budget and expanding its coverage for treatments related to blood disorders. Investments in medical staff and facilities will improve access to better diagnoses and treatments.
Creation of Novel Therapies: Research and development advances from throughout the world are finding their way to Indonesia, bringing with them more specialized and efficient therapies like gene therapy and customized medicine. To reduce expenses for the local people, there is a push to make generic and biosimilar versions of expensive medications more widely available.
Market Restraints
Accessibility and Affordability: A large number of necessary treatments for blood disorders come with a hefty out-of-pocket cost, particularly when it comes to novel gene therapies or foreign medications. Although JKN provides coverage for certain essential medications, there are still loopholes that require patients to pay for costly specialty medications. Rural communities have disadvantages due to the concentration of diagnosis, treatment, and specialized access in metropolitan regions.
Knowledge and Awareness Gaps: Ignorance about blood problems can cause stigma, poor treatment adherence, and delayed diagnosis. Timely intervention is hampered in many areas by a lack of knowledge about the early warning signs and symptoms of blood diseases. Education and outreach to people living in rural places are hampered by geographical and communication limitations. In rural regions, using traditional medicine is still common.
Limited Domestic Manufacturing and Research: The market is vulnerable to supply chain disruptions and foreign exchange volatility due to its reliance on overseas pharmaceutical businesses. The development of accessible and reasonably priced therapies that are suited to Indonesian requirements is hampered by a lack of local investment in research. Expanding the pool of skilled scientists and medical personnel is necessary to develop a strong domestic biopharmaceutical sector.
Healthcare Policies and Regulatory Landscape
Indonesia's commitment to enhancing public health and ensuring access to essential services is evident in its healthcare policies. However, achieving widespread coverage poses challenges due to the nation's diverse geography and large population. To address these issues, the National Health Insurance Program (JKN) was introduced, to offer affordable and comprehensive healthcare services to all citizens. The National Agency of Drug and Food Control (BPOM), Indonesia's drug regulatory body, plays a crucial role in upholding the safety and efficacy of pharmaceutical products. BPOM oversees the registration, licensing, and monitoring of drugs to ensure compliance with quality standards. Its pivotal function in conducting rigorous assessments of drug safety and efficacy before market approval is essential for safeguarding public health. Given the potential risks associated with substandard drugs, the role of BPOM becomes increasingly vital in preserving the well-being of the Indonesian population. The effective regulatory oversight by BPOM significantly contributes to the overall success and reliability of the healthcare infrastructure in the country.
Competitive Landscape
Key Players:
- Roche
- Novartis
- Pfizer
- Bayer
- BioMarin Pharmaceutical
- Takeda
- Kalbe Farma
- Dexa Medica
- Combiphar
- Kimia Farma
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Indonesia Blood Disorder Therapeutics Market Segmentation
By Disorder:
- Anemia
- Hemophilia
- Leukemia
- Myeloma
- Lymphoma
- Rare blood disorders
By Product Type
- Plasma-derived therapeutics
- Recombinant therapeutics
- Gene therapy
- Other therapies
By End User
- Hospitals
- Specialty clinics
- Ambulatory care
- Home healthcare
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.