Indonesia Biosensors Market Analysis
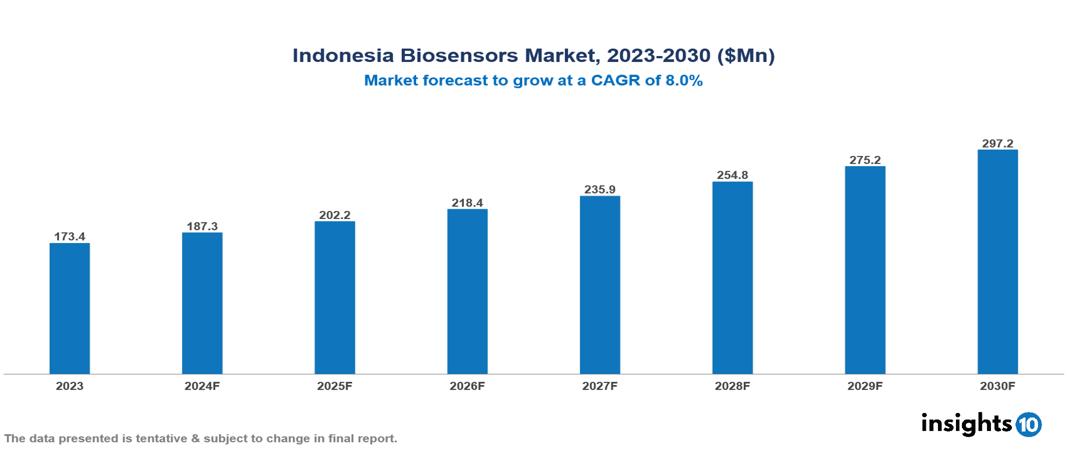
The Indonesia Biosensors Market was valued at $173.4 Mn in 2023 and is predicted to grow at a CAGR of 8% from 2023 to 2030, to $297.2 Mn by 2030. The key drivers of the market include increasing burden of chronic diseases, technological advancements, and growing demand for Point-of-Care (POC) testing. The prominent players of the Indonesia Biosensors Market are SD Biosensors, Meridian Bioscience, Biosensors International, Nix Biosensors, and Abbott Laboratories, among others.
Buy Now

Indonesia Biosensors Market Executive Summary
The Indonesia Biosensors market is at around $173.4 Mn in 2023 and is projected to reach $297.2 Mn in 2030, exhibiting a CAGR of 8% during the forecast period.
Biosensors, short for biological sensors, are analytical devices which combine a biological component with physicochemical detector in order to detect the presence of analytes in sample. There are certain static and dynamic attributes that every biosensor possesses. The optimisation of these properties is reflected on the performance of the biosensor. One of the most important features of a biosensor is selectivity. Selectivity is the ability of a bioreceptor to detect a specific analyte in a sample containing other admixtures and contaminants. Next crucial feature is the stability of biosensors which refers to how susceptible the biosensor is to internal and external disturbances. Due to disturbances, output signals can vary which may result in measurement errors in the concentration and can affect the biosensor’s accuracy and precision. Sensitivity is the minimum amount of analyte that can be detected by a biosensor which also defines its limit of detection (LOD). In a number of medical and environmental monitoring applications, a biosensor is required to detect analyte concentration of as low as ng/ml or even fg/ml to confirm the presence of traces of analytes in a sample. Linearity is the next attribute which represents the accuracy of the measured response with varying concentrations of analyte. Usually, a straight line in the form of equation y=mc (where c is the concentration of the analyte, y is the output signal, and m is the sensitivity of the biosensor) is used. Lastly, resolution is closely associated with linearity which is the smallest change in the concentration of an analyte which results in a change in the response of the biosensor.
Indonesia faces a significant challenge in managing the burden of chronic diseases on its healthcare system, which has directly led to increased rates of morbidity and mortality in the country. The Indonesia Biosensors Market is thus driven by significant factors such as the increasing burden of chronic diseases, technological advancements, and growing demand for Point-of-Care (POC) testing. However, high costs and limited reimbursement, technical challenges, and data privacy and security issues restrict the growth and potential of the market.
The leading players of the Indonesia Biosensors Market are SD Biosensors, Meridian Bioscience, Biosensors International, Nix Biosensors, and Abbott Laboratories, among others.
Market Dynamics
Market Growth Drivers
Increasing Burden of Chronic Diseases: The increasing cases of chronic diseases, such as diabetes, heart disease, and cancer, is drastically rising which often requires consistent monitoring to track disease progression and manage them effectively. The cancer incidence rate in Indonesia was 136.1 for 2022. Biosensors help in the continuous and real time evaluation of health parameters for patients such as glucose levels, heart rate, and oxygen saturation. This is extremely beneficial as it helps detect anomalies and results into timely intervention of medical care before the condition worsens. These advantages of biosensors are extremely vital for chronic conditions management which results in the growth of the Biosensors Market.
Technological Advancements: Advances in biosensor technology are continually progressing. The innovative application of fluorescence tagging and nanomaterials like graphene and carbon nanotubes has led to heightened sensitivity and improved detection limits. Using aptamers and nucleotides as recognition elements enhances the development of cutting-edge biosensor technologies. Additionally, nanotechnology, materials science, and microfabrication have enabled the creation of smaller, more sensitive, and faster-reacting biosensors. Biocompatible materials ensure wearable biosensors are comfortable and minimize body rejection. Moreover, integrating AI and ML algorithms enhances data analysis, enabling personalized healthcare and early disease detection. These technological advancements boost the performance of biosensors, driving market growth.
Growing Demand for Point-Of-Care (POC) Testing: The growing demand for Point-Of-Care (POC) testing is a significant driver for the biosensors market. Biosensors offer various benefits that conventional lab-based testing methods often lack, making them ideal for POC testing. These advantages include portability, user-friendliness, and quick results. Additionally, POC testing with biosensors enhances healthcare accessibility, even in remote locations or for patients with limited mobility. Consequently, the increasing demand for POC testing is fuelling the growth of the biosensors market.
Market Restraints
High Costs and Limited Reimbursement: The creation and production of biosensors are often expensive, which may inhibit innovation and result in high initial costs to cover production expenses. If biosensors do not prove to be more cost-effective than traditional methods, healthcare providers might be unwilling to invest in or recommend them. Additionally, insurance companies may not fully reimburse these expenses, limiting patient access to biosensor technologies. Consequently, the high costs and limited affordability of biosensors can prevent the market from growing.
Technical Challenges: Despite advancements in biosensors, technical challenges like biofouling and biocompatibility remain. Biofouling, the buildup of unwanted substances such as proteins, cells, and bacteria, reduces sensitivity, causes signal drift, complicates calibration, and damages sensors, thereby compromising biosensor performance. Biocompatibility issues are also significant, especially in medical applications, to prevent adverse tissue reactions. These challenges hinder the growth of the biosensors market.
Data Privacy and Security Concerns: Biosensor data contains highly sensitive personal information like patients' genetic data and health metrics, making it vital to prevent misuse, unauthorized access, and breaches. Increased interconnectivity of biosensors heightens their vulnerability to cyberattacks, such as data manipulation, operational disruptions, and theft of sensitive information. Protecting this data requires encryption and robust authentication and access control measures, which are both expensive and difficult to manage. These factors affect the overall growth of the biosensors market.
Regulatory Landscape and Reimbursement Scenario
In Indonesia, the regulatory agency is called the National Agency of Drug and Food Control (NADFC), also known by its Indonesian name, Badan Pengawas Obat dan Makanan (BPOM) which operates under the Ministry of Health. It plays a critical role in ensuring the safety, efficacy, and quality of pharmaceuticals available in the nation.
The NADFC conducts detailed review and analysis of the applications from pharmaceutical companies to register and market the new drugs. It includes a thorough evaluation of the safety, efficacy, and quality of pharmaceutical products. After the NADFC is ensured of these characteristics, does it allow the drugs to be marketed. The NADFC is also responsible for post-marketing surveillance of the drugs in case of any reporting of adverse drug reactions.
Indonesia’s healthcare reimbursement system is a complex mix of public and private insurance schemes. The mandated national health insurance scheme in Indonesia is called Jaminan Kesehatan Nasional (JKN), or National Health Insurance. It attempts to give access to basic healthcare and covers a sizable section of the populace. The JKN reimburses healthcare costs for outpatient and inpatient services at the designated healthcare facilities. For the private sector, compared to the JKN, private health insurance plans may provide greater coverage and better reimbursement rates.
Competitive Landscape
Key Players
Here are some of the major key players in the Indonesia Biosensors Market:
- SD Biosensor
- Medtronic
- Abbott Laboratories
- Johnson & Johnson
- Siemens Healthcare
- F. Hoffmann-La Roche
- Bio-Rad International
- Meridian Bioscience
- Nix Biosensors
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Indonesia Biosensors Market Segmentation
By Technology
- Electrochemical Biosensors
- Optical Biosensors
- Piezoelectric Biosensors
- Thermal Biosensors
- Nanomechanical Biosensors
By Product
- Wearable Biosensors
- Non-wearable Biosensors
By Application
- Medical Diagnostics
- Food Safety
- Environmental Monitoring
- Agriculture and Bioreactor Monitoring
- Other
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































