India Gene Editing Market Analysis
India Gene Editing Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Gene Editing is expanding as a result of rising demand for synthetic genes in both developed and developing nations. This demand is fueling the development of better devices and methods for the gene editing. Some of the key players in the global gene editing Market include Addgene, Allele Biotech, Bio-Rad, Takara Bio, CRISPR Therapeutics, OriGene Technologies, Precision Biosciences, GE Healthcare, Merck KGaA, and ThermoFischer Scientific Inc.
Buy Now

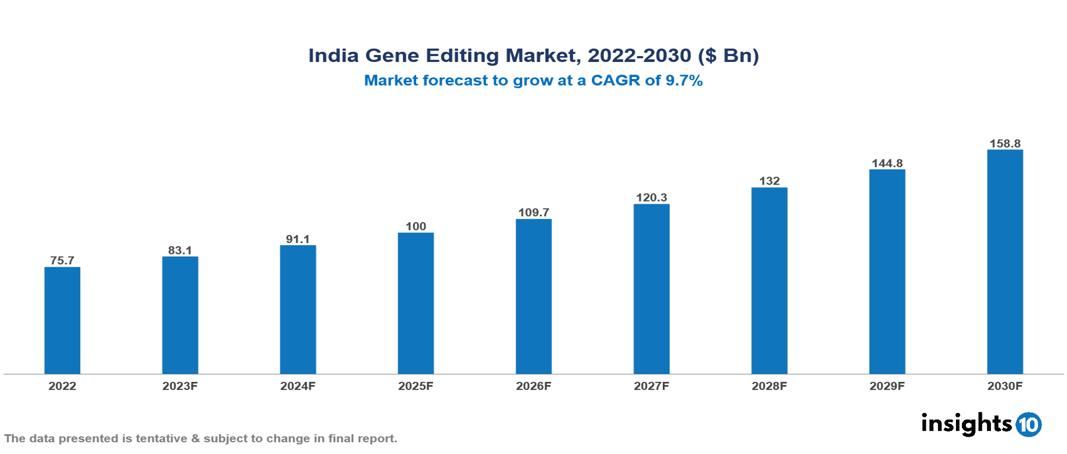
India Gene Editing Market Executive Summary
India Gene Editing Market is valued at around $75.7 Bn in 2022 and is projected to reach $158.7 Bn by 2030, exhibiting a CAGR of 9.7% during the forecast period 2023-2030.
Gene Editing Market refers to the market to change the genome of an organism. It is used to correct genetic defects, formation of new genes, or remove some of the genes. It is a very potential technique to revolutionize the healthcare sector to treat diseases and prevent diseases related to genetics.
The increasing prevalence of genetic disorders has contributed to the growth of the global market for gene editing in recent years. According to WHO approximately 1 in 20 people worldwide are affected by genetic disorders. Most common genetic disorders include Down syndrome, cystic fibrosis, sickle cell anemia, thalassemia, fragile X syndrome, and autism spectrum disorder. All of these disorders are increasing with time and driving the gene editing market.
There are many global players in the gene editing market but Addgene, Allele Biotech, Bio-Rad, Takara Bio, CRISPR Therapeutics, OriGene Technologies, Precision Biosciences, GE Healthcare, Merck KGaA, and ThermoFischer Scientific Inc. are some of the major players in the market.
Investments in research and development of gene editing therapy have increased the growth of the market. After the approval of CRISPR-Cas9, the growth of the market is boosted and other technologies like TALENs, base editing, prime editing, and gene therapy has been updated for effective and improved patient outcome.
The increasing prevalence of genetic disorders, investments in research and development, and advancement in technology are projected to fuel the market for gene editing in the coming years. The market still has many challenges including the high cost of treatment, ethical issues, complex regulatory procedures, limited knowledge, and the potential risk of gene editing. Continuous research, ethical discussions, and regulatory framework may help in addressing these issues and it can play a major role in gene editing therapy market growth which can be beneficial for the market and society as well.
Market Dynamics
Drivers of India Gene Editing Market:
Growing Incidence and Prevalence of Genetic Disorder: The prevalence of genetic disorders is increasing with every passing year and it is increasing the demand for treatment of genetic disorders. According to WHO around 2-5% of infants are detected with genetic disorders or abnormalities of all live births worldwide. Gene editing is needed an hour and it is growing the demand for gene editing market.
Advancement in Technology: There has been new developments and improvements in gene editing therapy and it has increased the efficiency and accuracy of therapy including improved patient outcome. Technologies like CRISPR-Cas9, base editing, and prime editing has been advanced with time and are driving the growth of the gene editing market.
Increasing Research and Development Investment: The market for gene editing is expanding as a result of rising investment in research and development in both developed and developing nations. This demand is fueling the development of better instruments and methods for gene editing therapy. It is driving a market for gene editing therapy.
Restraints of India Gene Editing Market:
High Cost of Treatment: Higher cost of gene editing treatment is the major restraint for the patients who need the gene editing treatment. The cost of gene editing therapy can vary depending on which type of gene therapy is needed and the severity of the patient’s condition. Gene therapy is much more expensive compared to conventional treatment for genetic disorders.
Ethical Considerations: When germline editing is done it raises a lot of ethical questions like unintended consequences, long-term effects of changing the germline, and undesired effects on future generations. Some people are concerned about creating “designer babies” which can alter the genetics of human beings which is not ethical.
Regulatory Framework: The process for entry into the gene editing market is very complicated and it requires a lot of time, resources, and money. Process for regulations may vary depending upon the countries. Some countries have very strict and extensive testing and clinical trials to evaluate safety and efficiency standards. It helps prevent unsafe practices but also makes it difficult to enter the market.
Public Acceptance: Gene editing technologies are still facing public resistance and it is a major factor that can restrict the growth of the gene editing market. For effective and fair deployment of gene editing treatment, there is a need to arrange a public discussion and social agreement on what applications are appropriate. It can help to create awareness and acceptance in public.
Key players
Agilent Technologies India Pvt Ltd Advinus Therapeutics Syngene International Ltd MedGenome Thermo Fisher Scientific India Ocimum Biosolutions Bharat Biotech Reliance Life Sciences Labindia Instruments Pvt Ltd Invitrogen Life Technologies India Pvt Ltd1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For India Gene Editing Market
By Technology:
- Zinc Finger Nucleases (ZFNs)
- CRISPR-Cas9 Gene Editing
- Restriction Enzymes
- Transcription Activator-Like Effector-based Nucleases (TALENs)
- Others
By Application:
- Drug development
- Gene editing
- Cell Line Engineering
- Animal Genetic Engineering
- Plant Genetic Engineering
- Others
By End-User:
- Biotechnology and Pharmaceutical Companies
- Academic and Government Research Institutes
- Contract Research Organizations
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































