India Bispecific Antibody Market Analysis
India Bispecific Antibody Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Bispecific Antibody is expanding as a result of the increasing prevalence of cancer and auto-immune diseases. This demand is fueling the development of novel bispecific antibody formats and technologies. Some of the key players in the global Bispecific Antibody Market include Roche Holding AG, Amgen Inc., Genentech, Inc., Regeneron Pharmaceuticals, Inc., Merus N.V., Xencor, Inc., Bristol-Myers Squibb Company, Novartis AG, Teneobio, Inc., and MacroGenics, Inc.
Buy Now

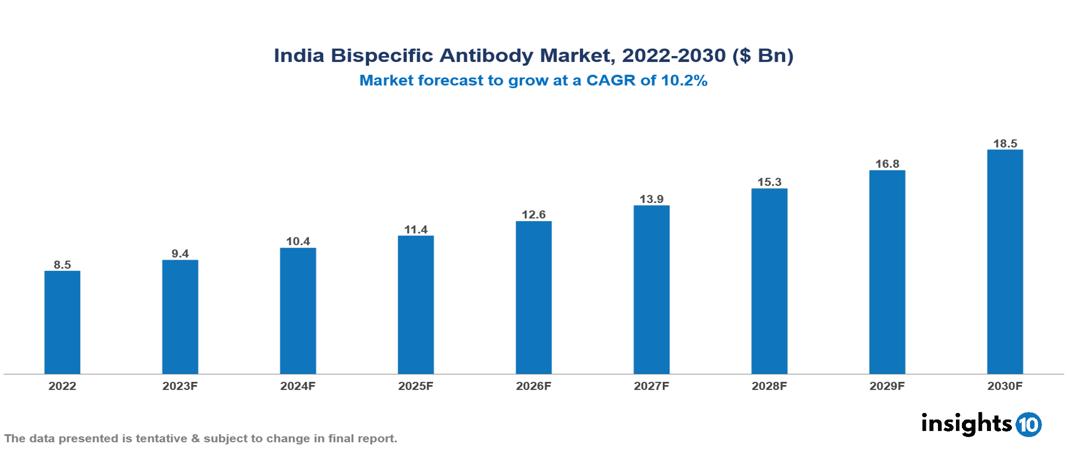
India Bispecific Antibody Market Executive Summary
India Bispecific Antibody Market is valued at around $8.5 Bn in 2022 and is projected to reach $18.4 Bn by 2030, exhibiting a CAGR of 10.2% during the forecast period 2023-2030.
The Bispecific Antibody Market refers to the market for the treatment of diseases like cancer and autoimmune diseases with bispecific antibodies, it features two antigen-binding sites within a single molecule. Bispecific antibodies come in various shapes and sizes, from huge IgG-like molecules with extra domains to small proteins with two linked antigen-binding fragments. The therapeutic success of currently available bispecific antibodies has sped up the development of these antibodies in oncology.
The increasing prevalence of cancer and autoimmune disorders has contributed to the growth of the global market for bispecific antibodies in recent years. According to WHO, there were around 50 Mn cases of autoimmune disorders and cancer was the second leading cause of the death. Advancements and new developments in bispecific antibodies for the treatment of these diseases are driving the market.
There are many companies in the global bispecific antibody market but Roche Holding AG, Amgen Inc., Genentech, Inc., Regeneron Pharmaceuticals, Inc., Merus N.V., Xencor, Inc., Bristol-Myers Squibb Company, Novartis AG, Teneobio, Inc., and MacroGenics, Inc. are some of the major players in the market.
Bispecific Antibodies can be used in the treatment of different diseases, including cancer, autoimmune disorders, and infectious diseases. It plays a crucial role in the development of novel and effective treatments for improving patient outcomes. For example, Blinatumomab is a bispecific antibody that targets CD19 and CD3 has been approved for the treatment of B-cell acute lymphoblastic leukemia, and is also being evaluated for the treatment of hematologic malignancies.
The increasing prevalence of chronic diseases and funding in research and development is projected to fuel the market for bispecific antibodies. The market still has many challenges like high development and commercialization costs, complex regulatory procedures, and immunogenic concerns.
Market Dynamics
Drivers of India Bispecific Antibody Market:
Growing Incidence and Prevalence of Chronic Diseases: The prevalence of chronic diseases like cancer, autoimmune disorders, and infectious diseases is increasing with every passing year. It is driving a demand for a more effective and innovative therapeutic approach. Bispecific antibodies have improved treatment outcome and it has the potential to fulfill unmet medical needs. This is raising the demand for the bispecific antibody market.
Advancement in Biotechnology: Technological innovations like antibody engineering and protein modification techniques have enabled the improved therapeutic potential of bispecific antibodies. It has also improved the precise targeting and therapeutic mechanism of bispecific antibodies. This is fueling the demand for the global bispecific antibody market.
Increasing Investment in Research and Development: Around 120 large, mid-sized, and small pharma companies can be found in the research and development of bispecific antibodies. In contrast to the over 180 candidates in the preclinical stages of development, over 220 bispecific antibodies are now either approved or being studied in the clinical stages of development. Additionally, there are many companies that are involved in contract manufacturing and research. Increasing investment in research and development has driven the growth of the market.
Restraints of India Bispecific Antibody Market:
Complex Manufacturing Procedure: Bispecific antibodies frequently need complicated production procedures, which can be problematic in terms of product quality consistency, cost-effectiveness, and scalability. The manufacture of bispecific antibodies must be reproducible and robust, so it is important to optimize the manufacturing procedures.
High Cost: The development and production of bispecific antibodies involve a huge amount of investment in research, preclinical and clinical trials, and infrastructure for manufacturing. It can be a restraint for smaller research organizations and biotechnology companies.
Complex Regulatory Procedures: The development and commercialization of bispecific antibodies require different types of regulatory licenses and approval for clinical and preclinical trials. Other types of standards for safety, efficacy, and quality should be followed to complete the regulatory procedures which can be challenging and time-consuming.
Immunogenicity Concerns: Bispecific antibodies can generate immune responses in patients which can lead to safety concerns and reduced efficacy. It can be challenging to manage such incidents and it can cause hurdles to the growth of the bispecific antibody market.
Notable Deals in Bispecific Antibody Market:
In March 2023, Akeso Biopharma declared that enrolment for a phase III trial of the drug Cadonilimab, which is to be used as first-line therapy for advanced gastric or gastroesophageal junction cancer when combined with chemotherapy, has been completed. It is also important to note that the target indications where bispecific antibodies have demonstrated their efficacy have been broadened with the most recent approval of TecvayliTM for the treatment of relapsed or refractory multiple myeloma.
Key players
Biocon Limited Glenmark Pharmaceuticals Limited Zydus Cadila Healthcare Limited Syngene International Ltd. Sun Pharmaceutical Industries Ltd. Dr. Reddy's Laboratories Ltd. Lupin Limited Intas Pharmaceuticals Ltd. Alkem Laboratories Ltd. Aurobindo Pharma Limited1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For India Bispecific Antibody Market
By Indication:
- Oncology
- Autoimmune Disease
- Others
By Drugs:
- Blinatumomab
- Catumaxomab
- Duligotumab
- Others
By Route of Administration:
- Intravenous
- Subcutaneous
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































