Germany Oncology Clinical Trials Market Analysis
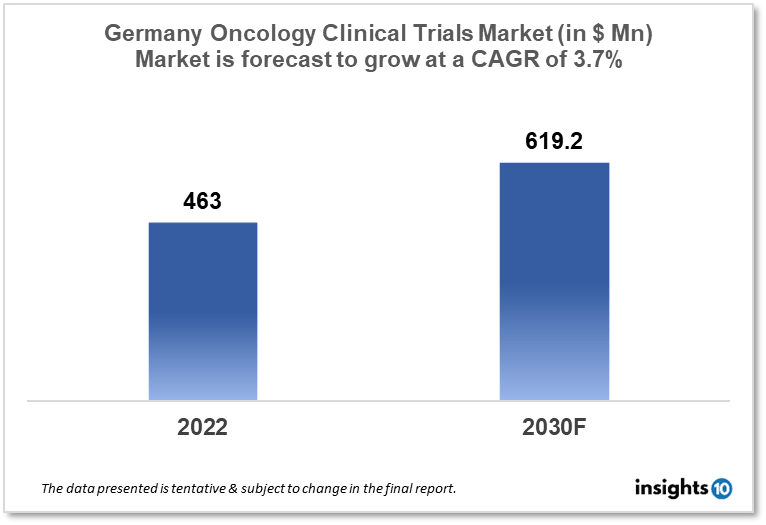
Germany's oncology clinical trials market is projected to grow from $463 Mn in 2022 to $619.2 Mn by 2030, registering a CAGR of 3.7% during the forecast period of 2022-30. The market will be driven by the strong healthcare infrastructure, focus on personalized medicine, and experienced workforce. The market is segmented by phase, by study design & by indication. Some of the major players include Novartis AG, Roche Holding AG, Bayer AG & BioNTech SE.
Buy Now

Germany Oncology Clinical Trials Market Executive Summary
The Germany Oncology Clinical Trials Market is projected to grow from $463 Mn in 2022 to $619.2 Mn by 2030, registering a CAGR of 3.7% during the forecast period of 2022 - 2030. The German Clinical Trials Registry (DRKS) is the major WHO registry in Germany. It is in charge of registering all patient-oriented clinical studies done in Germany. According to the WHO International Clinical Trials Registry Platform, In the year 2020, the number of clinical trials performed in Germany was 39,580 (24.22% of the total). Cancers of the breast, prostate, colorectum (big bowel), lung, and bladder are the most prevalent in Germany. According to Globocan 2020, the number of new cases of cancer in Germany was 6,28,519 with Breast cancer contributing to 11.1%, prostate cancer at 10.8%, and Lung cancer at 10.3% of the same.
Germany is a very appealing country for cancer clinical trials owing to its modern healthcare system, highly qualified workforce, and supportive regulatory environment. The country's emphasis on customized treatment and participation in novel clinical trials also makes it a significant participant in the worldwide cancer research scene. Germany is home to several of Europe's top cancer research institutions, including the;
- German Cancer Research Center (DKFZ)
- National Center for Tumor Diseases (NCT)
- Charité Comprehensive Cancer Center (CCCC)
Germany adheres to the EU Clinical Trial Regulation, which assures that all clinical studies adhere to stringent ethical and scientific criteria.
In October 2022, the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a favorable opinion recommending the approval of Pluvicto, a radioligand treatment used in the early stages of prostate cancer.
Market Dynamics
Market Growth Drivers
Many reasons are driving the expansion of cancer clinical trials in Germany. The country's superior healthcare system and solid infrastructure, which create a suitable environment for clinical research, are two major factors. Germany also has a huge patient population, making it a desirable site for clinical trials with high enrolment rates. Moreover, the nation offers a highly trained workforce, including many experienced clinical researchers, which contributes to its desirability as a clinical trial venue. The country's emphasis on individualized medicine in cancer therapy is another factor fuelling the expansion of oncology clinical trials in Germany. This strategy necessitates the creation of novel, focused medicines, which often involve clinical trials. Germany's research institutes and pharmaceutical businesses are working hard to develop these medicines and put them through clinical trials to ensure their safety and effectiveness.
Market Restraints
There are certain constraints to the expansion of cancer clinical trials in Germany. One of the most significant concerns is increased competition from other European nations, such as the United Kingdom and France, who are also actively investing in cancer research. Another concern is the complicated regulatory framework, which may often cause delays in clinical study approval.
Competitive Landscape
Key Players
- Pfizer Inc.
- Novartis AG
- Roche Holding AG
- AstraZeneca
- Merck & Co., Inc.
- Bristol-Myers Squibb Company
- Johnson & Johnson
- BioNTech SE (DEU)
- Bayer AG (DEU)
- Boehringer Ingelheim International GmbH (DEU)
Notable Insights
- In October 2022, the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) issued a favorable opinion recommending the approval of Pluvicto by Novartis, a radioligand treatment used in the early stages of prostate cancer.
- October 2021, BioNTech SE stated that the first colorectal cancer patient has been treated in a Phase 2 clinical study with their customized mRNA cancer vaccine BNT122
- July 2021, Valo Therapeutics Limited (ValoTx), announced the submission of a clinical trial application (CTA) to the German Regulatory Agency, The Paul Ehrlich Institute (PEI), for a Phase I, first-in-human trial of PeptiCRAd-1 (Peptide-coated Conditionally Replicating Adenovirus 1) in three tumor types.
- In June 2021, the first patient has been dosed in BioNTech's Phase II study of an mRNA cancer vaccine
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Clinical Trials Regulation in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
6. Methodology and Scope
Oncology Clinical Trials Market Segmentation
By Phase (Revenue, USD Billion):
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design Outlook (Revenue, USD Billion):
- Epilepsy
- Parkinson's Disease (PD)
- Huntington's Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle regeneration
- Others
By Indication Outlook (Revenue, USD Billion):
- Interventional
- Observational
- Expanded Access
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.










































