Germany Multiple Myeloma Therapeutics Market Analysis
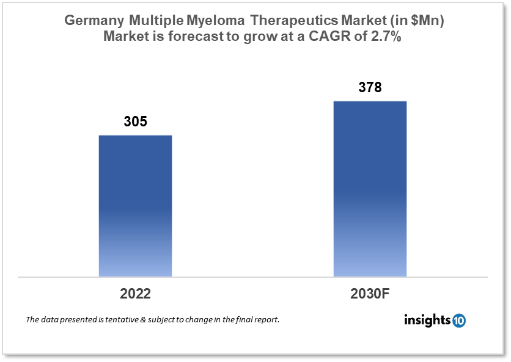
Germany's Multiple Myeloma Therapeutics Market was valued at $305 Mn in 2022 and is estimated to expand at a CAGR of 2.7% from 2022-30 and will reach $378 Mn in 2030. One of the main reasons propelling the growth of this Market is an increase in chronic disease, Increasing adoption of combination therapies. The Market is segmented by type, drug and distribution channel. Some key players in this Market are Abbott, MorphoSys, Merck, Medigene, Affimed, Helios Hospital, Vivantes Hospital Group and others.
Buy Now

Germany Multiple Myeloma Therapeutics Market Executive Summary
Germany's Multiple Myeloma Therapeutics Market was valued at $305 Mn in 2022 and is estimated to expand at a CAGR of 2.7% from 2022-30 and will reach $378 Mn in 2030. Multiple myeloma and plasma cell neoplasms are malignant growths of plasma cells that produce antibodies. This type of cancer typically begins in the bone marrow, where it frequently forms several disease foci (multiple myeloma) with consequences such as bone fractures, discomfort, and blood count aMnormalities. Around 1% of cases are identified in organs other than bone marrow (extramedullary plasmacytoma). In Germany, multiple myeloma was diagnosed in approximately 2,949 women and 3,741 men in 2019. It is highly uncommon for a disease to be diagnosed before the age of 45, as the risk rises dramatically with age (about 1.5 percent of all cases). Current incidence and mortality rates among women and men have remained relatively stable when adjusted for age.
Market Dynamics
Market Growth Drivers
The incidence of multiple myeloma in Germany has been steadily increasing in recent years, which is driving demand for new and innovative therapeutics. Multiple myeloma is more prevalent in older adults, and as the population in Germany continues to age, the demand for multiple myeloma therapeutics is expected to increase. There have been significant advancements in the development of new drugs for multiple myeloma in recent years, including immunomodulatory drugs and monoclonal antibodies, which are driving growth in the market. Combination therapies, which involve the use of multiple drugs to treat multiple myeloma, have become increasingly common in Germany, as they have been shown to be more effective than single-agent therapies. The German healthcare system provides favorable reimbursement policies for cancer treatments, including multiple myeloma therapeutics, which is helping to drive growth in the market. There are several promising drugs in the pipeline for the treatment of multiple myeloma, which are expected to drive growth in the market in the coming years.
Market Restraints
The German government and health insurers are placing increasing pressure on drug manufacturers to reduce prices, which is limiting the profitability of the market. There are several established and new players in the multiple myeloma therapeutics market, which is increasing competition and limiting pricing power. The regulatory approval process for new drugs in Germany can be time-consuming and expensive, which is limiting the availability of new and innovative treatments. Several key drugs used in the treatment of multiple myeloma are set to lose patent protection in the coming years, which is expected to increase competition from generic drugs and reduce revenues for branded drugs. While the German healthcare system provides universal coverage, access to specialized cancer centers and treatments may be limited in some areas, particularly in rural regions. Some multiple myeloma therapeutics are associated with adverse events and side effects, which can limit patient compliance and reduce demand for the drugs.
Competitive Landscape
Key Players
- MorphoSys
- Merck
- Medigene
- Affimed
- Helios Hospital
- Vivantes Hospital Group
- Asklepios St. Georg Hospital
Healthcare Policies and Regulatory Landscape
The German healthcare system is based on statutory health insurance, which covers the vast majority of the population. This means that the cost of multiple myeloma therapeutics is largely borne by health insurers. Drug prices are negotiated between pharmaceutical companies and the National Association of Statutory Health Insurance Funds (GKV-SV). These negotiations can be lengthy and are aimed at ensuring that prices are appropriate based on the clinical benefits of the drug. There are a number of cost containment measures in place in Germany, including reference pricing, which sets a maximum reimbursement price for drugs in a particular class, and rebates, which require drug manufacturers to pay a portion of their revenues back to health insurers.
Reimbursement Scenario
New treatments for multiple myeloma are subject to benefit assessments by the Federal Joint Committee (G-BA), which evaluates the additional benefit of new treatments compared to existing therapies. Based on this assessment, a drug may be classified as having an "added benefit", "no added benefit", or "less benefit" compared to existing treatments. This classification can impact the level of reimbursement for the drug. Once a drug has been approved and classified by the G-BA, the price of the drug is negotiated between the pharmaceutical company and the National Association of Statutory Health Insurance Funds (GKV-SV). The goal of these negotiations is to set a fair and reasonable price for the drug based on the clinical benefits it provides. The reimbursement rates for multiple myeloma treatments are based on the diagnosis-related group (DRG) system, which assigns a fixed reimbursement amount for each patient based on their diagnosis. The reimbursement rates for multiple myeloma treatments are typically higher than for other conditions due to the complexity and cost of treating this disease.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Multiple Myeloma Therapeutics Market Segmentation
By Treatment
- Chemotherapy
- Immunotherapy
- Targeted therapy
- Radiation therapy
- Stem cell transplant
By Distribution Channel
- Hospitals
- Retail
- Online
By End User (Revenue, USD Bn):
- Hospitals
- Clinics
- Cancer Research Centers
By Drug Class
Immunomodulatory drugs (IMiDs)
One of the most common therapies used to treat myeloma are immunomodulatory drugs, or IMiDs. These drugs work by modifying the immune system to attack myeloma cells and have been a significant advance in the treatment of multiple myeloma. Some of the commonly used IMiDs in multiple myeloma include:
- Lenalidomide (Revlimid): Lenalidomide is an oral medication that is used in the treatment of multiple myeloma, as well as other blood cancers such as myelodysplastic syndromes (MDS). It may help the immune system in eliminating cancerous or abnormal blood cells. Moreover, it might stop the development of new blood vessels that tumours require to expand.
- Pomalidomide (Pomalyst): Pomalidomide is an oral medication that is used to treat multiple myeloma. It works in a similar way to lenalidomide, by enhancing the immune system's ability to target cancer cells. Also, it makes it easier for immune cells to eliminate faulty myeloma cells, aiding in the production of healthy blood cells in the myeloma.
- Thalidomide (Thalomid): Thalidomide was the first IMiD to be used in the treatment of multiple myeloma. It works by modulating the immune system and preventing the growth of blood vessels that supply the tumor
- CC- 4047 (Actimid): CC-4047 is a newer IMiD that is still under investigation for the treatment of multiple myeloma. It works by inhibiting the growth of cancer cells and promoting their death.
Proteasome inhibitors
Proteasome inhibitors are a class of drugs that are commonly used in the treatment of multiple myeloma. They work by blocking the activity of proteasomes, which are cellular structures that break down proteins. This leads to the accumulation of proteins within the myeloma cells, ultimately causing their death. Some proteasome inhibitors used in multiple myeloma include:
- Bortezomib (Velcade): Bortezomib was the first proteasome inhibitor approved for the treatment of multiple myeloma. It is given by injection and is often used in combination with other drugs, such as chemotherapy or immunomodulatory drugs. Bortezomib is highly effective in inducing remissions in newly diagnosed and relapsed/refractory multiple myeloma patients.
- Carfilzomib (Kyprolis): Carfilzomib is a newer proteasome inhibitor that is approved for the treatment of multiple myeloma. It is given by injection and can be used as a single agent or in combination with other drugs. Carfilzomib has shown excellent results in heavily pretreated patients with relapsed/refractory multiple myeloma.
- Ixazomib (Ninlaro): Ixazomib is an oral proteasome inhibitor that is approved for use in combination with lenalidomide and dexamethasone for the treatment of multiple myeloma. It is the first oral proteasome inhibitor and offers the convenience of home administration.
Monoclonal antibodies:
Monoclonal antibodies, target specific proteins on the surface of myeloma cells, causing them to be destroyed by the immune system. Some of the commonly used monoclonal antibodies in multiple myeloma include:
- Daratumumab (Darzalex): Daratumumab is a monoclonal antibody that targets a protein called CD38, which is highly expressed on the surface of myeloma cells. By binding to CD38, daratumumab triggers the immune system to attack and destroy the cancer cells. It is approved for use in multiple myeloma in combination with other drugs, such as lenalidomide or bortezomib.
- Elotuzumab (Empliciti): Elotuzumab is a monoclonal antibody that targets a protein called SLAMF7, which is also expressed on the surface of myeloma cells. By binding to SLAMF7, elotuzumab enhances the immune system's ability to attack the cancer cells. It is approved for use in combination with lenalidomide and dexamethasone for the treatment of multiple myeloma.
- Isatuximab (Sarclisa): Isatuximab is a monoclonal antibody that targets a protein called CD38, similar to daratumumab. By binding to CD38, isatuximab triggers the immune system to attack and destroy the cancer cells. It is approved for use in combination with pomalidomide and dexamethasone for the treatment of multiple myeloma.
Chemotherapy drugs
Chemotherapy drugs work by killing rapidly dividing cells, including cancer cells. Chemotherapy is often used in combination with other drugs, such as steroids, immunomodulatory drugs, or proteasome inhibitors, to improve their effectiveness. Here are some chemotherapy drugs used in multiple myeloma:
- Melphalan: Melphalan is an alkylating agent that is commonly used in the treatment of multiple myeloma. It is used as a preventative measure before having a stem cell transplant to treat multiple myeloma. It works by damaging the DNA of cancer cells, leading to their death. Melphalan is often used in combination with other drugs, such as prednisone, to treat newly diagnosed multiple myeloma.
- Cyclophosphamide: Cyclophosphamide works by damaging the DNA of cancer cells, leading to their death. Cyclophosphamide is often used in combination with other drugs, such as dexamethasone, to treat relapsed or refractory multiple myeloma. Cyclophosphamide inhibits cancer cell proliferation, causing the body to kill the cancer cells.
- Doxorubicin: Doxorubicin works by inhibiting the synthesis of DNA and RNA, leading to the death of cancer cells. Doxorubicin is often used in combination with other drugs, such as bortezomib or dexamethasone, to treat newly diagnosed or relapsed/refractory multiple myeloma. Doxorubicin belongs to the anthracycline class of chemotherapeutic drugs, which also includes daunorubicin, idarubicin, and epirubicin.
- Etoposide: Etoposide is a topoisomerase inhibitor chemotherapy drug that is used in the treatment of multiple myeloma. It works by inhibiting the activity of topoisomerase enzymes, which are necessary for DNA replication and repair. Etoposide is often used in combination with other drugs, such as cisplatin, to treat relapsed or refractory multiple myeloma. It works by reducing or preventing cancer cell proliferation in your body.
Steroids
Steroids such as dexamethasone and prednisone are often used in combination with other drugs to treat multiple myeloma. They can reduce inflammation, suppress the immune system, and promote the death of myeloma cells.
- Dexamethasone: Dexamethasone works by reducing inflammation and suppressing the immune system. Dexamethasone is often used in combination with other drugs to treat relapsed or refractory multiple myeloma.
- Prednisone: Prednisone works by suppressing the immune system and reducing inflammation. Prednisone is often used in combination with other drugs, such as chemotherapy, to treat newly diagnosed multiple myeloma.
- Methylprednisolone: Methylprednisolone is a steroid that is similar to prednisone and is also used in the treatment of multiple myeloma. It works by suppressing the immune system and reducing inflammation. Methylprednisolone is often used in combination with other drugs to treat relapsed or refractory multiple myeloma.
Steroids are effective in reducing inflammation and suppressing the immune system, which can help to control the growth of myeloma cells. However, they can have side effects, such as weight gain, mood changes, and increased risk of infection, so their use needs to be carefully monitored.
Others
Other drug classes used to treat multiple myeloma include:
- Histone deacetylase inhibitors- A new class of cytostatic drugs that suppress tumour cell proliferation in vitro and in vivo by inducing cell cycle arrest, differentiation, and/or apoptosis
- Immune checkpoint inhibitors- Checkpoint proteins are produced by some immune system cells, such as T cells, as well as some cancer cells. These checkpoints prevent too aggressive immune responses and, in some cases, prevent T cells from destroying cancer cells.
- Targeted therapies- Proteasome inhibitors are one type of targeted therapy for multiple myeloma. Proteasome inhibitors include bortezomib (Velcade), carfilzomib (Kyprolis), and ixazomib (Ninlaro). They target proteasomes, which are enzymes that breakdown proteins in cells.
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































