Germany Mouth Ulcer Therapeutics Market Analysis
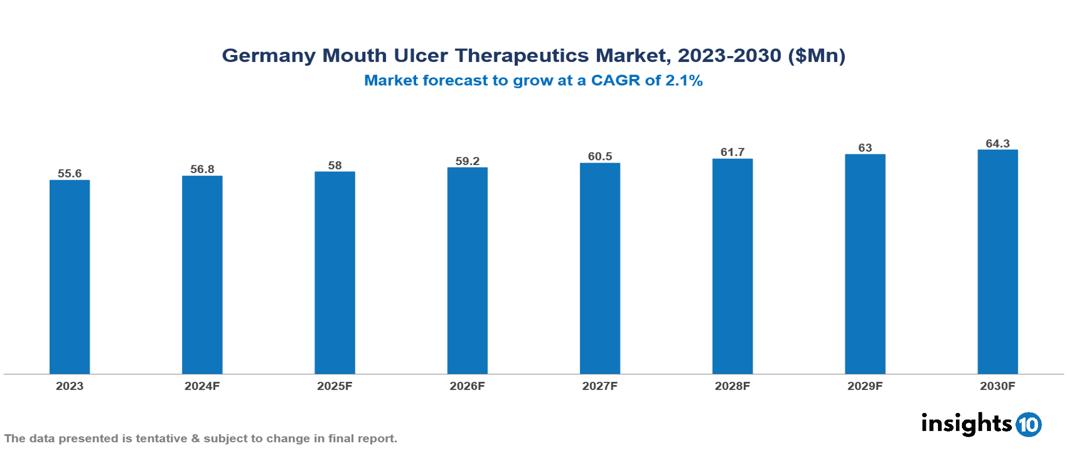
The Germany Mouth Ulcer Therapeutics Market was valued at $55.6 Mn in 2023 and is projected to grow at a CAGR of 2.1% from 2023 to 2023, to $64.3 Mn by 2030. The key drivers of this industry are rising awareness about oral hygiene, increasing demand for rapid healing products, growing geriatric population, rise in tobacco consumption, increasing incidence of oral diseases, chemical-based toothpaste and unhealthy lifestyles etc. The industry is primarily dominated by players such as Merck KGaA, Bayer, Boehringer Ingelheim, Sandoz, among others.
Buy Now

Germany Mouth Ulcer Therapeutics Market Executive Summary
The Germany Mouth Ulcer Therapeutics Market is at around $55.6 Mn in 2023 and is projected to reach $64.3 Mn in 2030, exhibiting a CAGR of 2.1% during the forecast period 2023-2030.
Mouth ulcers, also known as canker sores, are classified into three main types: minor aphthous ulcers which are the most common small oval lesions, major aphthous ulcers that are larger and deeper causing more pain, and herpetiform ulcers which start as multiple small ulcers that can merge into one large ulcer. While the exact cause is unknown, several risk factors have been identified including stress, anxiety, hormonal changes, nutritional deficiencies (iron, vitamin B12, folic acid, zinc), certain medical conditions like celiac disease, Crohn's disease, and HIV/AIDS, as well as trauma from dental work, braces, or ill-fitting dentures. In most cases, mouth ulcers are self-limiting and resolve within 1-2 weeks without treatment. However, complications can arise such as secondary infection, severe pain affecting one's ability to eat or drink properly, and potential scarring in cases of severe recurrent ulcers. Prevention involves reducing stress, avoiding potential food triggers like citrus fruits, nuts, and chips, using soft-bristled toothbrushes and waxed dental floss, and replacing toothpaste if it seems to be a trigger. Treatment involves symptomatic relief with topical gels containing anaesthetics, antiseptics, and antibiotics, as well as supportive care with multivitamins or vitamin supplements, antipyretics, and antibiotics. Medications include antibiotics, oral painkillers, oral steroids, antiseptics, chlorhexidine gluconate mouthwash, and sucralfate. Home care includes good oral hygiene, avoiding trigger foods, warm saline gargles, ice packs, and a balanced diet. Medical attention is necessary if the ulcers fail to heal within a week or two, are unusual, spreading, or large, or if there is severe pain, fever, or difficulty while chewing, talking, or swallowing.
The prevalence of oral mucosal lesions in Germany found that the overall prevalence was 7.76%, with the most common lesions being tobacco-associated lesions, tongue lesions, oral potentially malignant disorders, ulcers, and infectious lesions. The market therefore is driven by significant factors like the increasing incidence of mouth ulcers, rising awareness about oral hygiene, and unhealthy lifestyle habits, however, factors such as limited approved drugs, and challenges in patient compliance and treatment adherence limit market growth.
The key players of the market are Sandoz, Hexal, Reckitt Benckiser, Takeda, Bayer, Boehringer Ingelheim, Merck KGaA among others.
Market Dynamics
Market Growth Drivers
Increased occurrence of mouth ulcers: The prevalence of oral mucosal lesions in Germany found that the overall prevalence was 7.76%, the increasing prevalence of mouth ulcers in Germany is driven by factors such as smoking cessation, tobacco use, and consumption of citric and chemical-laden foods. This rising prevalence is expected to lead to a higher demand for effective treatments, driving market growth.
Unhealthy Lifestyles: The growing prevalence of unhealthy lifestyles, such as poor diet and lack of exercise, is also contributing to the rise in mouth ulcers. These factors can weaken the immune system, making individuals more susceptible to mouth ulcers.
Public Awareness Campaigns: Public awareness campaigns by governments and key players in the market are promoting the importance of oral hygiene and the availability of effective treatments for mouth ulcers. These campaigns are increasing awareness and driving demand for treatments.
Market Restraints
Limited Approved Drugs: In Germany, the treatment options for oral aphthous ulcers (mouth ulcers) are limited to a few approved drug categories. Corticosteroids, topical antiseptic/anti-inflammatory agents, and local anaesthetics are the primary pharmaceutical treatments available. The lack of a wider range of approved drugs may limit the treatment choices for healthcare professionals and patients, potentially hindering market growth.
Challenges in Patient Compliance and Adherence: Patient compliance and adherence to prescribed treatments can impact the effectiveness of oral ulcer management. Factors such as ease of use, side effects, and affordability of treatments can influence patient adherence. Addressing patient compliance challenges may drive the development of more user-friendly and convenient treatment options, shaping market trends.
Regulatory Landscape and Reimbursement Scenario
The Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) is an independent federal higher authority within the portfolio of the Federal Ministry of Health, and is responsible for the authorization, supervision, and post-market surveillance of drugs and medical devices. The BfArM also works closely with other regulatory agencies in Europe to facilitate the approval of new therapeutic products. Germany has several advantages for pharmaceutical companies, such as its market size, reliable legal framework for approval and reimbursement, as well as the quality and cost-effectiveness of its clinical research. Germany has the world’s fourth largest market. The German market is Europe’s largest market. Germany ranks third worldwide in clinical drug trials initiated by pharmaceutical companies.
Health insurance is mandatory for German citizens, Germany has approximately 83 Mn inhabitants. 73.2 Mn citizens are covered by the GKV (Gesetzliche Krankenversicherung = statutory health insurance), around 8.7 Mn are covered by the PKV (Private Krankenversicherung = private health insurance) including citizens are insured via state aid. GKV is provided by around 100 statutory health insurance funds.
The reimbursement system in Germany is complex and involves several steps. Initially, pharmaceutical companies set the ex-factory price for their products for a period of 12 months after market launch. After this period, the reimbursement price is negotiated based on the product's additional benefit to the patient compared to an appropriate comparative therapy. This assessment is conducted by the Institute for Quality and Efficiency in Health Care (IQWiG) or third parties.
The German government has also implemented measures to strengthen local production of medicines in Germany and the EU. This includes considering local production as a factor in the evaluation of new AMNOG provisions. The AMNOG market access rules apply to all new patented medicines introduced in the German market, except those with a conditional marketing authorization. These rules involve a benefit assessment process to determine the reimbursement price based on the product's additional benefit to the patient.
The reimbursement system in Germany is designed to ensure that pharmaceutical products are safe, effective, and affordable for patients in the country.
Competitive Landscape
Key Players
Here are some of the major key players in the Germany Mouth Ulcers Therapeutics Market:
- Bayer
- Boehringer Ingelheim
- Merck KGaA
- Takeda
- Sanofi
- Hexal
- Taro Pharmaceutical Industries Ltd.
- Bristol-Myers Squibb Company
- BLISTEX, INC.
- GlaxoSmithKline plc.
- Reckitt Benckiser
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Mouth Ulcer Treatment Market Segmentation
By Treatment Drug Class
- Corticosteroid
- Antihistamine
- Antimicrobial
- Analgesic
- Anesthetic
- Anti-inflammatory Agents
By Treatment Formulation Type
- Gel
- Mouthwash
- Ointment
- Spray
- Lozenges
Mouth Ulcer Treatment Indications
- Aphthous Stomatitis
- Oral Lichen Planus
- Others
By End Users
- Pharmacy
- Online Stores
- Hospitals and Clinics
- Home care
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































