Germany Liver Diseases Therapeutics Market Analysis
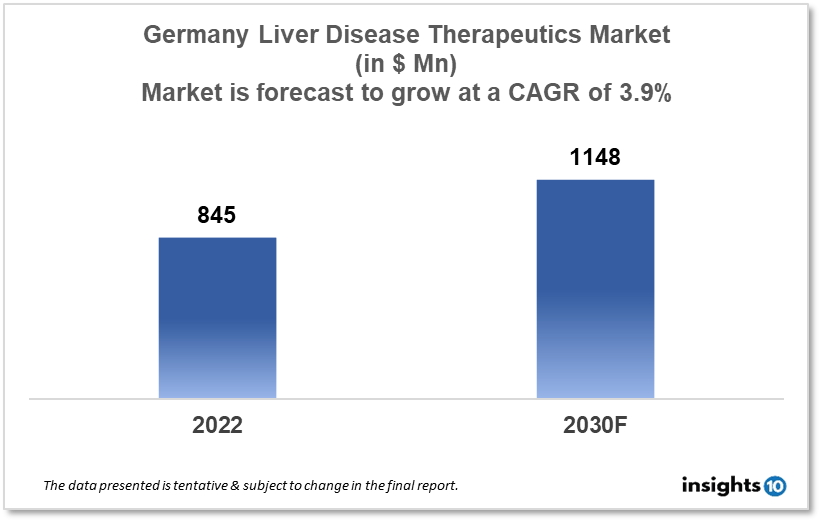
By 2030, it is anticipated that the Germany Liver Disease Therapeutics market will reach a value of $1148 Mn from $845 Mn in 2022, growing at a CAGR of 3.9% during 2022-2030. Liver Disease Therapeutics in Germany is dominated by a few domestic pharmaceutical companies such as Boehringer Ingelheim, Bayer, and BioNTech. The Liver Disease Therapeutics market in Germany is segmented into different types of disease and different therapy types. Some of the major factors affecting the German Liver Disease Therapeutics market are the growing prevalence of liver diseases and unhealthy and dynamic lifestyles.
Buy Now

Germany Liver Disease Therapeutics Analysis Summary
By 2030, it is anticipated that the Germany Liver Disease Therapeutics market will reach a value of $1148 Mn from $845 Mn in 2022, growing at a CAGR of 3.9% during 2022-2030.
Germany is a high-income, developed country located in the heart of Western Europe. According to the most recent WHO data, 15,194 people died from liver disease in Germany in 2020, accounting for 2.17 % of all deaths. With an age-adjusted death rate of 9.69 per 100,000 inhabitants, Germany ranks 128th in the world. Cirrhosis of the liver has the highest fatality rate of any chronic condition requiring hospitalisation in Germany.
Non-alcoholic fatty liver disease (NAFLD) and alcohol-related liver disease (ALD) are the most common kinds of liver disease in Germany. NAFLD is estimated to afflict up to 30% of the adult population in Germany, whereas ALD accounts for up to 80% of all liver disease cases in the country. Germany's government spent 12.8 % of its GDP on healthcare in 2020.
Market Dynamics
Market Growth Drivers Analysis
In Germany, the biggest risk factors for liver disease are obesity and excessive alcohol intake. Other risk factors include viral hepatitis, diabetes, and toxic exposure. In Germany, liver disease is a major public health issue. According to research, liver illness is the country's fifth leading cause of mortality, accounting for 2.6 % of all fatalities in 2019. The German government has taken many steps to address the country's liver disease problem, including education and preventative campaigns, increased research funding, and the development of dedicated liver centres. Germany has minimal structural unemployment and a robust apprenticeship programme. These aspects could boost the German Liver Disease Therapeutics market.
Market Restraints
End-stage liver disease is commonly treated by liver transplantation. However, the scarcity of liver donors poses a significant barrier to the treatment of liver illnesses. Patients with severe liver illnesses have fewer therapeutic options as a result of this. Despite immigration, Germany's labour force is expected to shrink beginning in 2020. These factors may deter new entrants into the German Liver Disease Therapeutics market.
Competitive Landscape
Key Players
- Boehringer Ingelheim: Boehringer Ingelheim is a German pharmaceutical company that specializes in the development of medicines for various diseases, including liver disease. They have developed a range of treatments for liver diseases such as non-alcoholic steatohepatitis (NASH), hepatitis B, and hepatitis C
- Bayer: Bayer is a multinational pharmaceutical company headquartered in Germany that also has a significant presence in the development of liver disease therapeutics. They have developed a number of treatments for liver diseases such as liver cancer and hepatitis C
- Merck: Merck is another German pharmaceutical company that is involved in the development of liver disease therapeutics. They have developed several treatments for liver diseases such as hepatitis B and hepatitis C
- BioNTech: BioNTech is a German biotechnology company that has gained worldwide recognition for developing the Pfizer-BioNTech COVID-19 vaccine. In addition to their COVID-19 vaccine work, they are also involved in the development of therapeutics for liver diseases such as hepatocellular carcinoma
- Affimed: Affimed is a German biopharmaceutical company that is focused on the development of immunotherapies for the treatment of cancer and other diseases, including liver cancer
- Fresenius Medical Care: Fresenius Medical Care is a German company that specializes in the production of dialysis equipment and the provision of dialysis services
Recent Notable Updates
June 2021: The US Food and Drug Administration (FDA) has given Fast Track Designation to Boehringer Ingelheim and Zealand Pharma's GLP-1/glucagon dual agonist BI 456906 for people with non-alcoholic steatohepatitis (NASH). Fast Track Designation expedites the development and review of novel treatments to treat serious illnesses that address an unmet medical need. A Phase II investigation of BI 456906 in people with NASH and liver fibrosis with and without diabetes is currently underway.
Healthcare Policies and Reimbursement Scenarios
In Germany, the regulatory authority for medicines is the Federal Institute for Drugs and Medical Devices (BfArM). The BfArM is responsible for the approval, regulation, and supervision of drugs in Germany. In Germany, healthcare is financed through a combination of statutory health insurance (SHI) and private health insurance (PHI). The reimbursement price for drugs in Germany is negotiated between the pharmaceutical company and the National Association of Statutory Health Insurance Funds (GKV-SV).
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Liver Disease Therapeutics Segmentation
By Treatment Type (Revenue, USD Billion):
- Chemotherapy
- Antiviral Drugs
- Vaccines
- Immunosuppressants
- Corticosteroids
- Immunoglobulins
- Targeted Therapy
By Disease Type (Revenue, USD Billion):
- Non-alcoholic Fatty Liver Disease (NAFLD)
- Autoimmune Diseases
- Cancer
- Hepatitis
- Genetic Disorder
- Others
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































