Germany Infectious Disease Therapeutics Market Analysis
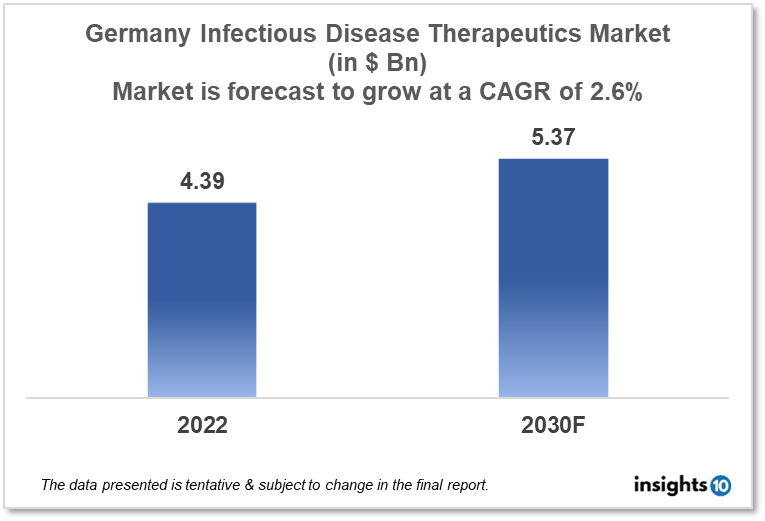
By 2030, it is anticipated that the German infectious disease therapeutics market will reach a value of $5.37 Bn from $4.39 Bn in 2022, growing at a CAGR of 2.6% during 2022-2030. Infectious Disease Therapeutics in Germany is dominated by a few domestic pharmaceutical companies such as BioNTech, Evotec and InfectoPharm. The Infectious Disease Therapeutics market in Germany is segmented into different therapeutic areas and different diseases type. The major factors affecting the German infectious disease therapeutics market are the increasing disease burden of communicable diseases like TB, hepatitis, and COVID-19 and the lack of healthcare funding for infectious disease treatment in various areas of Germany.
Buy Now

Germany Infectious Disease Therapeutics Analysis Summary
By 2030, it is anticipated that the German infectious disease therapeutics market will reach a value of $5.37 Bn from $4.39 Bn in 2022, growing at a CAGR of 2.6% during 2022-2030.
Germany is a high-income, developed country located in the heart of Western Europe. Immunotherapies, which use the body's immune system to combat infectious diseases, are increasingly gaining popularity in Germany. These medicines, which provide a tailored and successful approach to infectious illness therapy, are predicted to gain popularity in the future years. According to WHO figures, TB treatment coverage in Germany is roughly 61 %. In 2020, the leading notifiable infectious disease was coronavirus disease (COVID-19).
The COVID-19 pandemic not only imposed a massive strain on Germany's public health system, but it also influenced the occurrence and detection of other notifiable infectious diseases. The Infection Protection Act is continuously updated to reflect changes in the epidemiological situation in Germany and around the world. Germany's government spends 12.8 % of its GDP on healthcare.
Market Dynamics
Market Growth Drivers Analysis
While infectious illness rates in Germany have been relatively low in recent years, several infectious diseases, such as tuberculosis, hepatitis C, and sexually transmitted infections, have recently increased (STIs). This has increased the demand for effective treatments to treat these disorders. The German government is investing in infectious illness research, including the development of novel medicines and vaccinations. Germany has a low structural unemployment rate and a well-developed apprenticeship system These aspects could boost Germany's infectious disease therapeutics market.
Market Restraints
Germany has a strict drug clearance process, and the German regulatory body, the Federal Institute for Drugs and Medical Devices (BfArM), is well-known for its demanding safety and efficacy standards. This can make it difficult for pharmaceutical companies to get their infectious illness medicines approved and marketed in the country. While Germany has a robust healthcare system, it remains vulnerable to developing infectious diseases, which can pose new obstacles to pharmaceutical development and dissemination. Despite immigration, the working population is expected to decline beginning in 2020. These factors may deter new entrants into the German infectious disease therapeutics market.
Competitive Landscape
Key Players
- CureVac: CureVac is a biopharmaceutical company that specializes in the development of vaccines and immunotherapies. The company is based in Tübingen, Germany. CureVac is currently working on a COVID-19 vaccine
- BioNTech: BioNTech is a biotechnology company based in Mainz, Germany. The company focuses on the development of immunotherapies for cancer and infectious diseases. BioNTech is one of the companies that developed a COVID-19 vaccine in collaboration with Pfizer
- Evotec: Evotec is a drug discovery and development company based in Hamburg, Germany. The company focuses on the development of drugs for infectious diseases, including bacterial and viral infections
- AiCuris: AiCuris is a biotech company that develops drugs for viral and bacterial infections, including HIV, hepatitis B, and cytomegalovirus. The company is based in Wuppertal, Germany
- InfectoPharm: InfectoPharm is a German pharmaceutical company that specializes in the development of drugs for infectious diseases, including bacterial and viral infections
Recent Notable Updates
July 2021: The Global Antibiotic Research and Development Partnership (GARDP) and InfectoPharm are collaborating to discover improved treatment options for neonatal sepsis, a leading cause of mortality and impairment in babies up to 28 days old, using combinations of existing antibiotics. Because children of this age lack a functioning immune system, they are more susceptible to infection and less able to overcome it.
Healthcare Policies and Reimbursement Scenarios
The regulation of infectious disease therapies in Germany is the responsibility of the Federal Institute for Medications and Medical Devices (BfArM), which is in charge of reviewing and approving novel drugs and medical devices. The approval procedure for infectious disease treatments in Germany encompasses numerous steps, including preclinical testing, clinical trials, and marketing authorization. Before initiating clinical trials, the producer must submit an application to the BfArM, which will assess the proposed drug's safety and efficacy. If the application is granted, the firm will be able to begin clinical testing. Other regulatory agencies in Germany, in addition to the BfArM, may be involved in the regulation of infectious disease treatments. For example, the Paul Ehrlich Institute (PEI) is responsible for evaluating the safety and efficacy of vaccines and blood products.
The reimbursement of infectious illness treatments in Germany is essentially governed by the statutory health insurance system. This system covers more than 85 % of the German population, and drug reimbursement is overseen by the Federal Joint Committee (G-BA) and the Institute for Quality and Efficiency in Health Care (IQWiG).
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Infectious Disease Therapeutics Segmentation
By Mode of Treatment (Revenue, USD Billion):
- Vaccines
- Drugs
By Applications (Revenue, USD Billion):
- HIV/AIDS
- Influenza
- Hepatitis
- Malaria
- Tuberculosis
- Others
By Disease Type (Revenue, USD Billion):
- Viral Diseases
- Bacterial Diseases
- Fungal Diseases
- Parasitic Diseases
- Others
By Target Organism (Revenue, USD Billion):
- Antibiotics
- Antivirals
- Antifungals
- Anti-Parasitic
- Others
By End User (Revenue, USD Billion):
- Hospitals and Clinics
- Ambulatory Care Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.
The regulation of infectious disease therapies in Germany is the responsibility of the Federal Institute for Medications and Medical Devices (BfArM), which is in charge of reviewing and approving novel drugs and medical devices.
This system covers more than 85 % of the German population, and drug reimbursement is overseen by the Federal Joint Committee (G-BA) and the Institute for Quality and Efficiency in Health Care (IQWiG).
Infectious Disease Therapeutics in Germany is dominated by domestic pharmaceutical companies such as BioNTech, Evotec and InfectoPharm.







































