Germany Hepatitis A Therapeutics Market Analysis
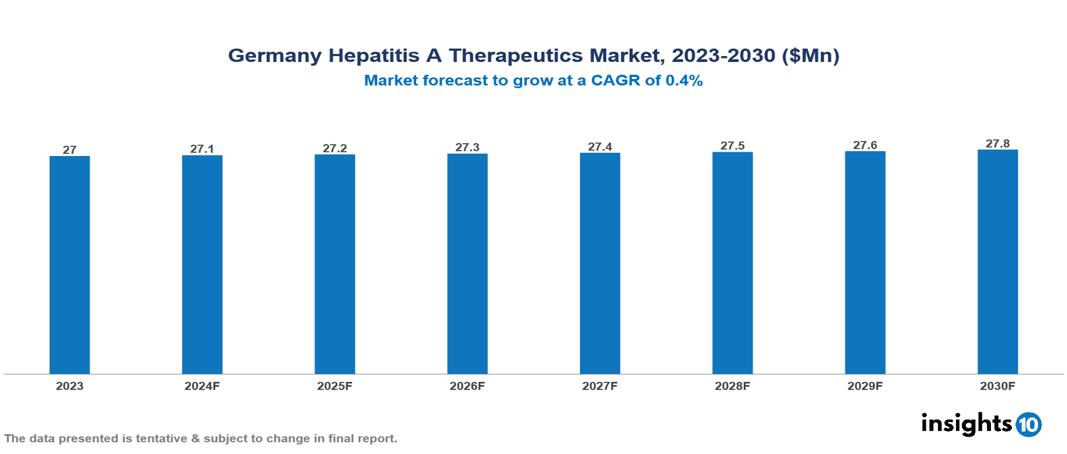
The Germany Hepatitis A Therapeutics Market was valued at $26.98 Mn in 2023 and is predicted to grow at a CAGR of 0.4% from 2023 to 2030 to $27.75 Mn by 2030. This market growth is driven by factors such as the rising prevalence of the disease, unmet vaccination needs, and the traveling population. Prominent companies in this sector include Sanofi and GlaxoSmithKline (GSK), among others.
Buy Now

Germany Hepatitis A Therapeutics Market Executive Summary
The Germany Hepatitis A Therapeutics Market was valued at $26.98 Mn in 2023 and is predicted to grow at a CAGR of 0.4% from 2023 to 2030 to $27.75 Mn by 2030.
Hepatitis A, a highly contagious viral infection causing liver inflammation, is primarily transmitted through contaminated food or water containing the Hepatitis A virus (HAV). While young children may be asymptomatic, older individuals typically develop mild flu-like symptoms and abdominal pain. Hepatitis, a condition characterized by liver inflammation, can arise from various factors like toxin exposure, alcohol misuse, immune disorders, or infections, with viruses like Hepatitis A being the primary cause. This acute form of hepatitis usually resolves without treatment. While Hepatitis A is typically a self-limiting illness, the ongoing transmission of the virus in and the rising number of reported cases underscore the importance of continued efforts to prevent and control Hepatitis A infections.
Germany reported 582 cases of Hepatitis A in 2022, the third-highest number in the EU/EEA region. Vaccination coverage for Hepatitis A is relatively low, with only 48.6% of the population. Factors such as the increasing disease prevalence, unmet vaccination needs, and traveling population drive market growth. In contrast, limited treatment availability, and regulatory hurdles restrain the market growth.
Market Dynamics
Market Growth Drivers
Rising Prevalence: Increasing prevalence of Hepatitis A infections: In 2022, Germany reported 582 cases of Hepatitis A, the third highest number in the EU/EEA region. This highlights the ongoing need for effective Hepatitis A treatments.
Unmet Vaccination Needs: Despite the availability of Hepatitis A vaccines, a significant portion of the German population remains unvaccinated, presenting an unmet need and a key driver for the Hepatitis A therapeutics market. Vaccination coverage for Hepatitis A is relatively low, with only 48.6% of the overall population having a prevalence of antibodies against the Hepatitis A virus (anti-HAV). This low vaccination coverage underscores the substantial market potential for Hepatitis A therapeutics as the unvaccinated population remains at risk for infection, thereby driving the demand for therapeutic interventions.
Growing Traveling population: Approximately 60% of Hepatitis A cases in Germany occur in individuals without a travel history, indicating a substantial unvaccinated population. Thus, travel is as a primary driver for the Hepatitis A therapeutics market.
Market Restraints
Limited Treatment Availability: The limited availability of specific hepatitis A treatments in certain healthcare facilities can be identified as a market restraint for the Germany Hepatitis A therapeutics market analysis. This constraint hinders the effective management and treatment of Hepatitis A within the healthcare system, potentially impacting patient outcomes and access to necessary medications, thus restraining the market.
Complex Regulatory Environment: The complex regulatory landscape in Germany, including rigorous marketing authorization, pricing policies, and health technology assessments, presents significant challenges for Hepatitis A therapeutics. Requirements for safety, quality, and effectiveness set by BfArM and benefit assessments by AMNOG and G-BA create barriers to market access. These processes are time-consuming and costly, potentially limiting market growth for Hepatitis A treatments in Germany.
Regulatory Landscape and Reimbursement Scenario
The reimbursement scenario for Hepatitis A therapeutics in Germany is favorable due to the country's universal healthcare system, funded through social security contributions. Various health insurance funds manage medication reimbursement, ensuring high coverage for Hepatitis A treatments to prioritize patient access. Coverage is typically more likely for high-risk individuals, such as unvaccinated or immunocompromised patients with clinically significant Hepatitis A infections.
Regulatory bodies like the Federal Institute for Drugs and Medical Devices (BfArM) and the Paul Ehrlich Institute (PEI) play crucial roles in ensuring the safety and efficacy of pharmaceutical products, including Hepatitis A therapeutics. Physicians may need to justify medication choices, especially in non-standard treatment situations. Patients usually do not directly manage reimbursement, with most costs covered by the healthcare system, although a small co-payment might be required.
Competitive Landscape
Key Players
Here are some of the major key players in the Hepatitis A Therapeutics Market:
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- Zydus Cadilla
- Sanofi
- GlaxoSmithKline (GSK)
- Takeda
- CVS Health Corporation
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Hepatitis A Therapeutics Market Segmentation
By Distribution Channel
- Hospital-based pharmacies
- Retail pharmacies
- Online pharmacies
By Route of Administration
- Oral Medications
- Intravenous Therapy
By Healthcare Setting
- Outpatient Care
- Inpatient Care
By Age
- Children
- Adults
- Senior Citizens
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































