Germany Exosome Research Market Analysis
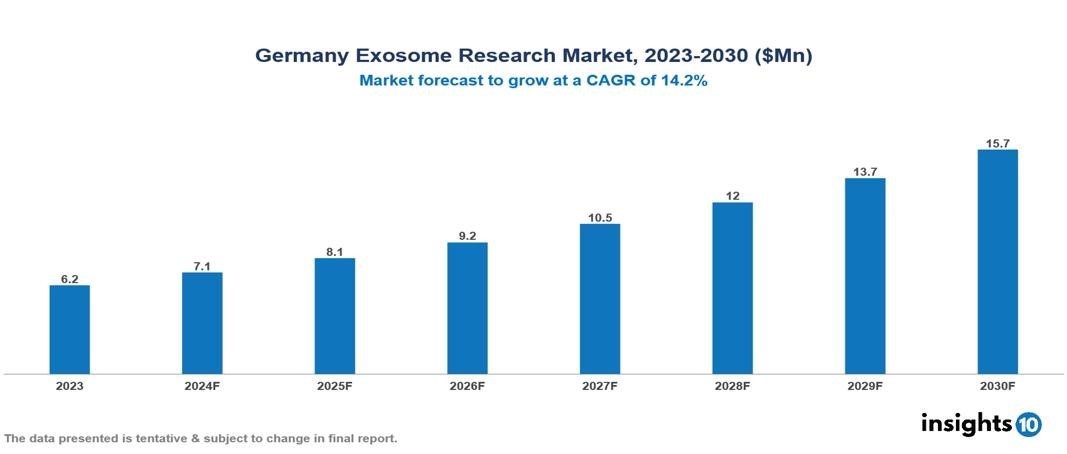
The Germany Exosome Research Market was valued at $6.2 Mn in 2023 and is projected to grow at a CAGR of 14.2% from 2023 to 2023, to $15.7 Mn by 2030. The key drivers of this industry are increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, and increasing advanced applications of exosomes. The industry is primarily dominated by players such as Thermo Fisher Scientific, NanoSomix, NX Pharmagen, Malvern Instruments, Capricor Therapeutic among others.
Buy Now

Germany Exosome Research Market Executive Summary
The Germany Exosome Research Market is at around $6.2 Mn in 2023 and is projected to reach $15.7 Mn in 2030, exhibiting a CAGR of 14.2% during the forecast period 2023-2030.
Exosomes are nano-sized extracellular vesicles released by most cell types, playing a crucial role in intercellular communication and the transportation of molecules like proteins, lipids, and nucleic acids. The exosome market has garnered significant attention due to its potential applications across various fields. In therapeutics, exosomes can be used as natural drug delivery vehicles to transport therapeutic molecules to target cells or tissues, potentially improving specificity and reducing side effects. Additionally, the molecular composition of exosomes can serve as biomarkers for various diseases, enabling early detection and monitoring. In the cosmeceuticals sector, exosomes derived from stem cells or other sources are being explored for skin rejuvenation and anti-aging products. Furthermore, exosomes are valuable research tools for studying intercellular communication, disease mechanisms, and developing new therapeutic strategies.
Exosomes have significant therapeutic potential, particularly in the context of COVID-19 and other diseases. They can be used as cell-free alternatives for treating various conditions and tissue regeneration by delivering therapeutic cargo components while avoiding immune rejection and cellular toxicity. Stem cell-derived exosomes are particularly advantageous in harnessing the anti-inflammatory and regenerative abilities of parent cells, making them suitable for engineered treatments for respiratory viral diseases like SARS-CoV-2. Exosomes also have promising potential as a vehicle for drug delivery due to their natural material transportation properties, ability to support intrinsic long-term circulation, and high biocompatibility. These factors make them suitable for delivering a variety of proteins, chemicals, and nucleic acids. Research studies have shown positive results for exosomes as mediators of intercellular communication, potentially delivering functional proteins, mRNA transcripts, and miRNAs to cells throughout the body. Additionally, exosomes derived from certain cell types, such as dendritic and mesenchymal stem cells, have therapeutic properties and are biocompatible and efficient agents against various disorders, including organ injury and conditions like heart, kidney, liver, and lung illnesses. These properties make exosomes a promising therapeutic platform for the treatment of a range of diseases.
According to the Global Cancer Observatory, an estimated 231,400 women and 261,800 men in Germany were newly diagnosed with cancer. The 5-year prevalent cases of cancer in Germany are approximately 505,000. The market therefore is driven by significant factors like increasing prevalence and incidences of cancer and various auto-immune diseases, rising healthcare expenditure for better health services, growing R&D activities associated with exosome research, technological advancements in exosome isolation and analytical procedures, and increasing advanced applications of exosomes.
Some of the major players operating in the German Exosome Research Market are Thermo Fisher Scientific, NanoSomix, NX Pharmagen, Malvern Instruments, and Capricor Therapeutics among others.
Market Dynamics
Market Drivers
Growing Demand for Cancer Diagnostics: The 5-year prevalent cases of cancer in Germany are approximately 505,000. The rising incidence of cancer in Germany is leading to a growing demand for cancer diagnostics, including exosomes-based tests. Exosomes are being explored as potential biomarkers for cancer diagnosis and monitoring, which can help in early detection and treatment.
Aging Population and Chronic Disease Burden: Germany has an aging population with a high prevalence of chronic diseases like cancer, cardiovascular disorders, and neurodegenerative diseases. Exosomes hold promise as diagnostic and therapeutic tools in these areas, driving market demand.
Government Funding and Initiatives: The German government provides funding and support for innovative research, including in the field of exosomes. Initiatives like the Federal Ministry of Education and Research's "NanoMatFutur" program can fuel exosome market growth.
Market Restraints
Regulatory Challenges: The regulatory landscape for exosome-based products in Germany and the European Union is complex and evolving. Navigating the approval processes for exosome-based diagnostics and therapeutics can be challenging, potentially hindering market growth.
Reimbursement and Pricing Issues: Obtaining appropriate reimbursement and pricing for exosome-based products from healthcare payers in Germany may pose challenges, as they are still considered novel and expensive technologies.
Technical Barriers: The isolation, purification, and characterization of exosomes can be technically complex and costly, requiring specialized equipment and expertise. These technical barriers may slow down exosome research and product development.
Regulatory Landscape and Reimbursement Scenario
The Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) is an independent federal higher authority within the portfolio of the Federal Ministry of Health, and is responsible for the authorization, supervision, and post-market surveillance of drugs and medical devices. The BfArM also works closely with other regulatory agencies in Europe to facilitate the approval of new therapeutic products. Germany has several advantages for pharmaceutical companies, such as its market size, reliable legal framework for approval and reimbursement, as well as the quality and cost-effectiveness of its clinical research. Germany has the world’s fourth largest market. The German market is Europe’s largest market. Germany ranks third worldwide in clinical drug trials initiated by pharmaceutical companies.
Health insurance is mandatory for German citizens, Germany has approximately 83 Mn inhabitants. 73.2 Mn citizens are covered by the GKV (Gesetzliche Krankenversicherung = statutory health insurance), around 8.7 Mn are covered by the PKV (Private Krankenversicherung = private health insurance) including citizens are insured via state aid. GKV is provided by around 100 statutory health insurance funds.
The reimbursement system in Germany is complex and involves several steps. Initially, pharmaceutical companies set the ex-factory price for their products for a period of 12 months after market launch. After this period, the reimbursement price is negotiated based on the product's additional benefit to the patient compared to an appropriate comparative therapy. This assessment is conducted by the Institute for Quality and Efficiency in Health Care (IQWiG) or third parties.
The German government has also implemented measures to strengthen local production of medicines in Germany and the EU. This includes considering local production as a factor in the evaluation of new AMNOG provisions. The AMNOG market access rules apply to all new patented medicines introduced in the German market, except those with a conditional marketing authorization. These rules involve a benefit assessment process to determine the reimbursement price based on the product's additional benefit to the patient. The reimbursement system in Germany is designed to ensure that pharmaceutical products are safe, effective, and affordable for patients in the country.
Competitive Landscape
Key Players
Here are some of the major key players in the German Exosomes Research Market:
- Thermo Fisher Scientific
- NanoSomix
- NX Pharmagen
- Malvern Instruments
- Capricor Therapeutic
- Exosome Diagnostics
- System Biosciences
- Exosome Sciences
- Aegle Therapeutic
- AMS Biotechnology
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Exosomes Research Market Segmentation
By Products
- Instruments
- Software
- Reagents and Kits
By Applications
- Diagnostics
- Therapeutic
By Indication
- Cancer
- Cardiovascular Disease
- Neurogenerative Disease
- Infectious Disease
- Others
By Key End Users
- Hospitals
- Cancer Institutes
- Diagnostic Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.