Germany Digital Biomarkers Market Analysis
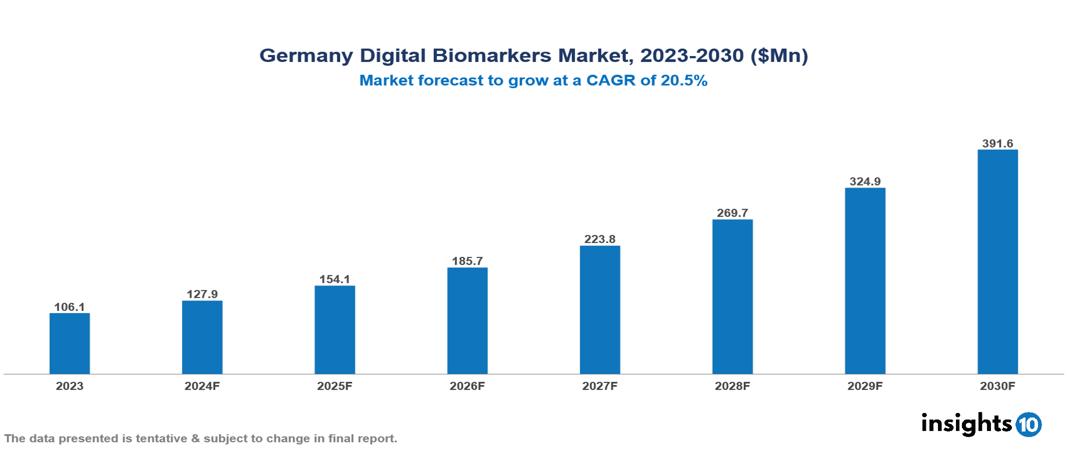
Germany Digital Biomarkers Market was valued at $106.14 Mn in 2023 and is predicted to grow at a CAGR of 20.5% from 2023 to 2030, to $391.55 Mn by 2030. The key drivers of this industry include advancements in technology, the growing prevalence of chronic diseases, and the focus on preventative care. The industry is primarily dominated by ActiGraph LLC, AliveCor Inc., Koneksa, and Altoida Inc. among others.
Buy Now

Germany Digital Biomarkers Market Executive Summary
Germany Digital Biomarkers Market was valued at $106.14 Mn in 2023 and is predicted to grow at a CAGR of 20.5% from 2023 to 2030, to $391.55 Mn by 2030.
Digital biomarkers are quantifiable physiological and behavioral data collected by digital devices like wearables and smartphones. They provide insights into health, including disease progression and treatment response, enabling continuous monitoring and real-time data collection. These biomarkers are used for various health conditions, such as diabetes, cardiovascular disorders, mental health issues, and neurodegenerative diseases. By tracking metrics like physical activity, heart rate, and sleep patterns, digital biomarkers help clinicians make informed decisions and support research with real-world evidence. As technology advances, their potential to transform healthcare and improve patient outcomes grows.
Chronic diseases are a major health concern in Germany, accounting for roughly 44% of the total disability-adjusted life years in the country (Nature Scientific Data, 2023). During the COVID-19 pandemic, market players introduced innovative solutions, such as a cost-effective mobile app by Maastricht University (September 2022) to detect COVID-19 via voice changes. The WHO noted over 59,000 active clinical trials globally in 2020, underscoring the rising demand for digital biomarkers.
The market therefore is driven by significant factors like advancements in technology, growing prevalence of chronic diseases, and the focus on preventative care. However, data privacy and security concerns, lack of standardization, and high development costs restrict the growth and potential of the market.
Prominent players in this field are ActiGraph LLC which specializes in wearable devices and data analytics for physical activity and sleep monitoring, contributing to clinical research through reliable digital biomarkers. AliveCor Inc. excels in portable ECG monitoring and AI-driven cardiac analysis, enhancing early diagnosis and intervention with FDA-cleared devices like KardiaMobile. Others contributors include Koneksa, and Altoida Inc. among others.
Market Dynamics
Market Growth Drivers
Advancements in Technology: The swift advancement of digital health technologies, such as wearable devices, smartphones, and sophisticated analytics, is propelling the use of digital biomarkers. Innovations in AI and machine learning are improving the precision and predictive capabilities of these biomarkers.
Growing Prevalence of Chronic Diseases: Germany faces rising chronic diseases like diabetes, cardiovascular issues, and neurodegenerative disorders, driving demand for digital biomarkers. These enable early detection, continuous monitoring, and personalized treatments, supporting market growth. Chronic diseases are a major cause of death and disability, with over two-thirds of Germans aged 65+ managing at least two chronic conditions, highlighting critical healthcare challenges.
Focus on Preventative Care: There's a growing emphasis on preventative healthcare in Germany Digital biomarkers can empower individuals to take a more proactive approach to their health by tracking health indicators and identifying potential risks early on.
Market Restraints
Data Privacy and Security Concerns: The collection and use of personal health data through digital biomarkers raise significant privacy and security concerns. Ensuring the protection of sensitive information and maintaining patient trust are major challenges.
High Development Costs: Developing and validating digital biomarkers involves substantial investment in technology, research, and clinical trials. These high costs can be a barrier for smaller companies and startups.
Lack of Standardization: Standardization in data collection, analysis, and interpretation of digital biomarker data is still under development. This inconsistency can limit the usability and interoperability of these technologies across different healthcare settings.
Regulatory Landscape and Reimbursement Scenario
Germany boasts a well-established regulatory framework overseen by the Federal Institute for Drugs and Medical Devices (BfArM), which governs medical devices, including digital biomarkers. BfArM employs a specific classification system for digital health technologies like digital biomarkers, ensuring they meet stringent standards for safety, effectiveness, and data privacy. Companies developing these technologies must secure a CE marking, indicating compliance with European Union (EU) regulations for medical devices. Additionally, adherence to Germany's robust data protection regulations under the Bundesdatenschutzgesetz (BDSG) is crucial, safeguarding patient data throughout its lifecycle—from collection and storage to security and user consent.
In terms of reimbursement, Germany offers a progressive landscape through its Social Health Insurance System (gesetzliche Krankenversicherung - GKV). Since 2021, Germany has implemented the DiGA initiative, which allows for the reimbursement of certain digital health applications, including those integrating digital biomarkers, by the GKV. This initiative underscores Germany's commitment to advancing digital health solutions that demonstrate clinical benefits and cost-effectiveness. Private health insurance companies in Germany also play a significant role, providing additional coverage options for digital health solutions incorporating digital biomarkers.
Competitive Landscape
Key Players
Here are some of the major key players in the Germany Digital Biomarkers Market
- ActiGraph LLC
- AliveCor Inc.
- Koneksa
- Altoida Inc.
- Amgen Inc.
- Biogen Inc.
- Empatica Inc.
- Vivo Sense
- IXICO plc
- Adherium Limited
1. Executive Summary
1.1 Digital Health Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Digital Health Policy in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Digital Biomarkers Market Segmentation
By Type
- Wearable
- Mobile-based Applications
- Sensors
- Others
By Clinical Practice
- Diagnostic digital biomarkers
- Monitoring digital biomarkers
- Predictive and Prognostic digital biomarkers
- Other's (Safety, Pharmacodynamics/ Response, Susceptibility)
By Therapeutic Area
- Cardiovascular and metabolic disorders (CVMD)
- Respiratory disorders
- Psychiatric disorders
- Sleep & Movement Disease
- Neurological disorders
- Musculoskeletal disorders
- Others (Diabetes, Pain Management)
By End-Use
- Healthcare companies
- Healthcare Providers
- Payers
- Others (Patients, caregivers)
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































