Germany Dental Endodontics Market Analysis
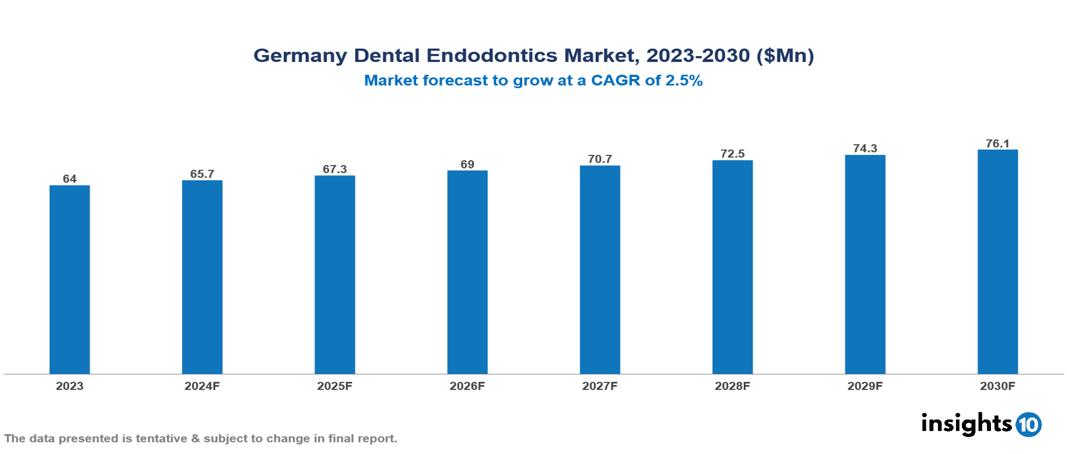
Germany Dental Endodontics Market was valued at $64 Mn in 2023 and is predicted to grow at a CAGR of 2.5% from 2023 to 2030, to $76.10 Mn by 2030. The key drivers of this industry include a high focus on oral health, an aging population, and technological advancement. The industry is primarily dominated by Coltene, Dentsply Sirona, FKG Dentaire, and Ultradent Products among others.
Buy Now

Germany Dental Endodontics Market Executive Summary
Germany Dental Endodontics Market was valued at $64 Mn in 2023 and is predicted to grow at a CAGR of 2.5% from 2023 to 2030, to $76.10 Mn by 2030.
The dental endodontics market plays a crucial role in preserving dental health by addressing diseases and injuries affecting the dental pulp. When this pulp becomes inflamed or infected due to factors such as dental decay, trauma, or infection, it can lead to severe pain, swelling, and ultimately, the loss of the affected tooth if left untreated. It encompasses a range of procedures, with the most common being root canal therapy. This vital treatment aims to save a damaged or infected tooth by removing the inflamed pulp tissue (the inner layer containing nerves and blood vessels) and disinfecting the canals within the tooth's root. In addition to root canal therapy, these may include procedures such as pulp capping, pulpotomy, and apexification, each tailored to address specific conditions or stages of pulp damage
In Germany, approximately 10% of all teeth have undergone root canal treatment, underscoring the importance of non-surgical root canal treatment (NSRCT) in general dental care. Dental caries is highly prevalent, affecting about 70% of various groups, including those with endodontically treated teeth. The market therefore is driven by significant factors like a high focus on oral health, an aging population, and technological advancement. However, stringent regulatory landscape, limited insurance coverage, shortage of dental specialists, and cost-effectiveness considerations restrict the growth and potential of the market.
Prominent players in this field are Coltene, who launched MicroMega One RECI (a single shaping file in reciprocating motion). This is expected to minimize the mechanical impact on the dental hard tissues and Dentsply Sirona, which is a global leader in dental technology, providing innovative solutions for endodontic procedures. Other contributors include FKG Dentaire and Ultradent Products among others.
Market Dynamics
Market Growth Drivers
High Focus on Oral Health: Germany's strong culture of preventative dental care promotes a population that values procedures like root canal therapy to preserve natural teeth, resulting in higher acceptance and demand for such treatments.
Aging Population: As Germany's population ages, with 22.1% aged 65 years and older in 2022 and projections showing this group growing to 24 Mn by 2050, there is an increasing prevalence of age-related dental issues. This demographic shift drives the demand for endodontic interventions to manage conditions like tooth decay and pulp inflammation effectively.
Technological Advancements: German dental practices are known for adopting advanced technologies such as digital imaging, 3D printing for custom restorations, and sophisticated rotary instrumentation. These technologies improve the accuracy, efficiency, and success rates of root canal procedures, making them more attractive to patients.
Market Restraints
Stringent Regulatory Landscape: Germany's strict regulatory framework for medical devices, including those used in endodontics, can result in higher costs for manufacturers and potentially slower market entry for innovative technologies, posing a challenge for market growth.
Limited Insurance Coverage: While Germany's well-developed public healthcare system provides extensive coverage, statutory health insurance plans (SHI) might not fully cover the costs of complex endodontic procedures. Patients with private dental insurance often have more comprehensive coverage, but out-of-pocket expenses can still be a concern.
Shortage of Dental Specialists: There may be a shortage of qualified endodontists, especially outside major cities, limiting access to specialized procedures in some regions and affecting the overall availability of advanced endodontic care.
Cost-Effectiveness Considerations: German healthcare providers and insurers may prioritize cost-effective solutions. Although advanced endodontic techniques offer long-term benefits, the upfront costs may be a barrier for some patients, potentially limiting the adoption of these procedures.
Regulatory Landscape and Reimbursement Scenario
In Germany, the regulation of dental medicines and devices used in endodontic procedures is overseen by the Federal Institute for Drugs and Medical Devices (BfArM). BfArM ensures that dental products meet rigorous safety and efficacy standards before they can be used in clinical settings. Additionally, the Federal Chamber of Dentists (Bundeszahnärztekammer - BZÄK) establishes professional standards and ethical guidelines for dental practitioners, including those specializing in endodontics. This regulatory framework is essential for maintaining high standards of dental care and protecting patient health by ensuring that dental professionals adhere to best practices.
Germany's statutory health insurance (SHI) covers a significant portion of the population and provides varying levels of coverage for endodontic procedures. Basic root canal treatments are generally covered, but more complex procedures that involve advanced techniques or materials might require co-pays or may not be fully covered. Conversely, many Germans also have private dental insurance plans that offer more comprehensive coverage for dental treatments, including endodontics. The extent of this coverage depends on the specific plan and insurer, with plans typically covering a substantial portion of the costs associated with root canal procedures. However, patients may still face out-of-pocket expenses depending on their plan details.
Competitive Landscape
Key Players
Here are some of the major key players in the Germany Dental Endodontics Market
- Dentsply Sirona
- Danaher Corporation
- Ivoclar Vivadent
- Ultradent Products, Inc.
- Septodont Holding
- FKG Dentaire
- Peter Brasseler Holdings, LLC
- Mani, Inc.
- Coltene Holding AG
- Henry Schein, Inc
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Dental Endodontics Market Segmentation
Instruments
- Endodontic scalers and lasers
- Motors
- Apex locators
- Machine-assisted obturation systems
- Others
Consumables
- Obturation
- Shaping and Cleaning
- Access Cavity Preparation
End Users
- Dental Hospital
- Dental clinics
- Dental academics and research institutes
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































