Germany Cough Hypersensitivity Syndrome Therapeutics Market Analysis
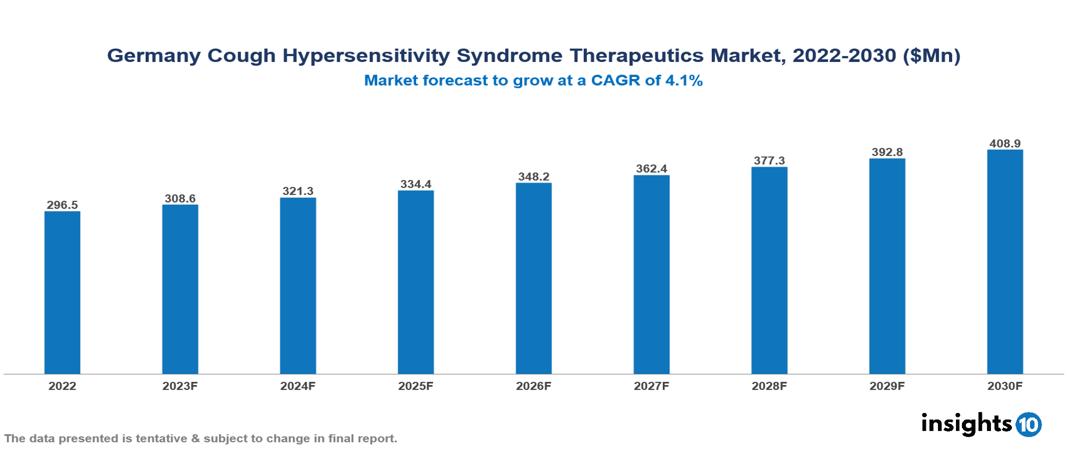
The Germany Cough Hypersensitivity Syndrome Therapeutics Market was valued at $296 Mn in 2022 and is predicted to grow at a CAGR of 4.1% from 2023 to 2030, to $409 Mn by 2030. The key drivers of this industry include the increasing prevalence of Cough Hypersensitivity Syndrome, expanding government initiatives, and high unmet medical needs. The industry is primarily dominated by players such as Roche, Pfizer, Teva, Novartis, Johnson & Johnson, and GSK among others.
Buy Now

Germany Cough Hypersensitivity Syndrome Therapeutics Market Analysis Executive Summary
The Germany Cough Hypersensitivity Syndrome Therapeutics Market is at around $296 Mn in 2022 and is projected to reach $409 Mn in 2030, exhibiting a CAGR of 4.1% during the forecast period.
An increased cough reflex in reaction to stimuli such as temperature changes, mechanical forces, or chemical exposure defines Cough Hypersensitivity Syndrome (CHS). CHS is associated with underlying diseases such as asthma, upper airway cough syndrome (UACS), gastroesophageal reflux disease (GERD), rhinitis, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis. Risk factors for CHS are higher sensitivity to stimuli such as citric acid or capsaicin. CHS treatment options include antitussive medications, inhaled corticosteroids (ICS), anticholinergics, and antihistamines, among others. Johnson & Johnson, Bayer AG, and Teva Canada are among the leading pharmaceutical companies developing CHS therapies.
Cough Hypersensitivity Syndrome contributed to around 20%-30% of the total chronic cough cases in Germany. Major contributors to market expansion include the rising prevalence of Cough Hypersensitivity Syndrome and subsequently its risk factors like smoking and pollution, expanding government initiatives, and high unmet medical needs. However, factors such as a lack of treatments and efficacy, diagnostic barriers, and reimbursement issues limit the market's growth and potential.
Market Dynamics
Market Growth Drivers
Increasing prevalence of Cough Hypersensitivity Syndrome: Chronic cough affects roughly 5-10% of the German population, with CHS accounting for 20-30% of all cases. The older population, with weaker immune systems, has increased sensitivity to respiratory infections and CHS, generating a patient pool that requires efficient therapies.
High unmet medical need: The therapeutic gap in treatments is driving the demand for accurate and efficient drugs. The growing interest in non-pharmaceutical treatments such as inhalers and cough hypersensitivity-specific immunotherapy is widening the spectrum of treatment options and adoption among patients.
Expanding government initiatives: The German government is dedicated to enhancing respiratory health outcomes and has introduced measures to enhance accessibility to healthcare services and make medications more affordable. These efforts include investment for the development of new CHS drugs, and initiatives to raise awareness and improve diagnosis. Germany's healthcare system, which includes specialized clinics and hospitals, allows the implementation of novel CHS medications.
Market Restraints
Limited treatment options and efficacy: Current treatments for CHS rely on the use of off-label drugs such as inhaled corticosteroids, antitussives, and others which have side effects and limited efficacy in long-term treatment. There is no specific drug approved for CHS in Germany which complicates focused treatment results in diagnostic delays, restricting market growth.
Diagnostic challenges: CHS exhibits symptoms similar to other respiratory conditions such as asthma and GERD, posing challenges in achieving accurate diagnosis and potentially resulting in misidentification and inappropriate treatment. The lack of well-defined and universally accepted diagnostic criteria adds complexity to the diagnostic process, impeding timely intervention.
Reimbursement challenges: The utilization of medications for CHS in ways not explicitly approved may not receive complete insurance coverage, imposing a financial strain on patients. If new therapies specifically formulated for CHS are introduced, the substantial costs associated with their development may result in elevated market prices, potentially restricting accessibility for certain patients.
Notable Updates
August 2021, Nocion Therapeutics, a clinical-stage biopharmaceutical firm focusing on the development of innovative small molecule charged sodium channel blockers known as nocions, specifically targeting actively firing nociceptors, has reported that the initial ten participants have received doses in the Phase 2a trial of an inhalable variant of NTX-1175. The study is currently enrolling participants in Germany.
Healthcare Policies and Regulatory Landscape
In Germany, the main regulatory body overseeing drugs and pharmaceuticals is the Federal Institute for Drugs and Medical Devices (BfArM), known in German as Bundesinstitut für Arzneimittel und Medizinprodukte. BfArM operates under the Federal Ministry of Health and is responsible for ensuring the safety, efficacy, and quality of pharmaceuticals and medical devices in the country. It evaluates applications for marketing authorization, monitors product safety throughout their lifecycle, and sets guidelines and standards for the pharmaceutical industry.
Obtaining a drug license in Germany requires submitting a comprehensive application to the BfArM including detailed information on the drug's quality, and efficacy. The proposal is evaluated, including scientific assessments and inspections of manufacturing facilities. If the drug meets regulatory standards, the BfArM grants marketing approval, allowing it to be sold in Germany
The regulatory environment for new entrants in the pharmaceutical industry is stringent but transparent, with clear guidelines and processes in place. Companies entering the market must demonstrate compliance with German and European Union regulations, ensuring that their products meet high standards of safety and efficacy before gaining approval for commercialization in Germany.
Competitive Landscape
Key Players
- Pfizer
- GlaxoSmithKline
- Boehringer Ingelheim
- AstraZeneca
- Johnson & Johnson
- Bayer
- Novartis
- Teva Pharmaceuticals
- Roche
- Merck
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Cough Hypersensitivity Syndrome Therapeutics Market Segmentation
By Drug Class
- Antitussive Agents
- Corticosteroids
- Short Acting Beta-2 Agonists
- Anti-cholinergic
- Antihistamines
- Proton Pump Inhibitors
- Others
By Route of Administration
- Oral
- Inhalation
- Others
By End Users
- Hospitals
- Speciality Clinics
- Homecare
By Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.