Germany Clinical Trial Planning and Design service Market Analysis
Germany Clinical Trial Planning and Design Service Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. The market for Clinical Trial Planning and Design Service Market is growing due to rapid increase in technological advancements, growing collaboration, and surge of vendors in the market. Some of the key players in the global Clinical Trial Planning and Design Service Market include PharmsLex, Emergo UL, Cytel, Health Policy Associate, CD BioSciences, LLX Solutions, SGS, ADM Korea, ClinAsis, BioPoint, Atlantia Clinical Trials, IQVIA, and PPD, Inc.
Buy Now

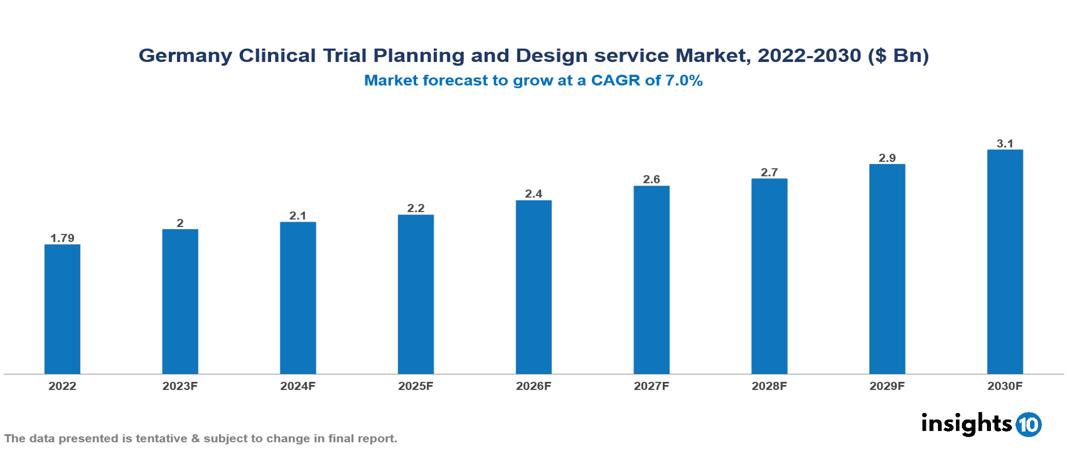
Germany Clinical Trial Planning and Design service Market Executive Summary
Germany Clinical Trial Planning and Design service Market is valued at around $1.79 Bn in 2022 and is projected to reach $3.08 Bn by 2030, exhibiting a CAGR of 7% during the forecast period 2023-2030.
The Clinical Trial Planning and Design Service provide data that is accurate, flexible, consistent, and compliant with clinical studies. When developing new drugs or doing molecular analyses on generic and biosimilar drugs, pharmaceutical companies use planning and design services for clinical trials. Market growth is anticipated to be boosted by factors like increased government spending in clinical trial protocol research and development, rising use of clinical trial management services, and adoption of cutting-edge medical technology. The market for Clinical Trial Planning and Design Services is expanding as a result of rising chronic and infectious illness prevalence, higher R&D spending by pharma and biotech businesses, and other factors.
Clinical Trials Planning and Design are essential components of the entire drug development process because they allow both innovators and regulators to evaluate the safety and efficacy of a therapeutic candidate. The fact that these studies account for about 50% of all time and financial investments made in the development of medication candidates further supports their crucial importance.
Pharmaceutical and biotechnology firms like PharmsLex, Emergo UL, Cytel, Health Policy Associate, CD BioSciences, LLX Solutions, SGS, ADM Korea, ClinAsis, BioPoint, Atlantia Clinical Trials, IQVIA, and PPD, Inc., are some of the major players in the Clinical Trial Planning and Design Service Market.
Conducting trials is frequently laden with difficulties, such as the complexity of the science and operations, issues with attracting and keeping qualified subjects, problems with data handling, and more strict regulatory standards.
The cost of a clinical trial's failure might be extremely high for the sponsors. Furthermore, it is thought that careful trial preparation can help to save a huge share of losses. In other words, efficient Clinical Trial Planning and Design is essential to ensure that the study is carried out across all sites in a timely, accurate, and safe manner.
Market Dynamics
Drivers of Germany Clinical Trial Planning and Design Service Market:
Technological Advancements: Players in the pharmaceutical industry have frequently used cutting-edge technologies to address current issues over the years. Similarly to this, clinical trial sponsors are investigating the latest platforms and technologies with the goal of further streamlining the entire procedure.
The surge in the Number of Vendors Providing Solutions for Clinical Trial Planning and Design: The number of suppliers providing a variety of services and solutions for clinical trial planning and design has recently increased in the clinical research sector as well.
Collaborations of Small Businesses: Collaborations between numerous startups and small businesses have been formed in an effort to progress their ideas and provide specialised research skills, services, and tools growing the market for Clinical Trial Planning and Design Services.
Focusing on the development of Software: In order to automate the process and enable effective planning and design of clinical trials, a number of service providers active in this field have moved their focus to software development.
Notable Deals in Clinical Trial Planning and Design Service Market:
In 2022, Cytel Inc.'s business extended across the Asia-Pacific area (APAC). This enables Cytel's existing biometrics and advanced statistical solutions to be more accessible to APAC biotech and biopharma enterprises. Cytel now has operations in Australia, Shanghai, Beijing, and Singapore, with plans to grow into Seoul and Tokyo in the future. This expansion is the latest move in Cytel's goal to provide advanced analytics capabilities to drug developers worldwide.
In 2022, Parexel announced the opening of a new clinical trial supplies and logistics facility in Suzhou, China. This strategically positioned facility offers local and international biopharmaceutical companies conducting clinical trials in the region quick access to supplies and investigational therapies to distribute to clinical locations and patients worldwide.
Key players
IQVIA PRA Health Sciences ICON plc Covance Inc. Syneos Health PAREXEL International Corporation Charit? Research Organisation GmbH ClinAssess GmbH Medpace Holdings, Inc. Celerion Germany GmbH1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For Germany Clinical Trial Planning and Design Service Market
By Service:
- Statistical Analysis Plan
- eCRF
- Site Identification and Selection
- Medical Writing
- Others
By Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
By Therapeutic Area:
- Cardiovascular Disorders
- Inflammatory Disorders
- Neurological Disorders
- Oncological Disorders
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































