Germany Cholesterol Therapeutics Market Analysis
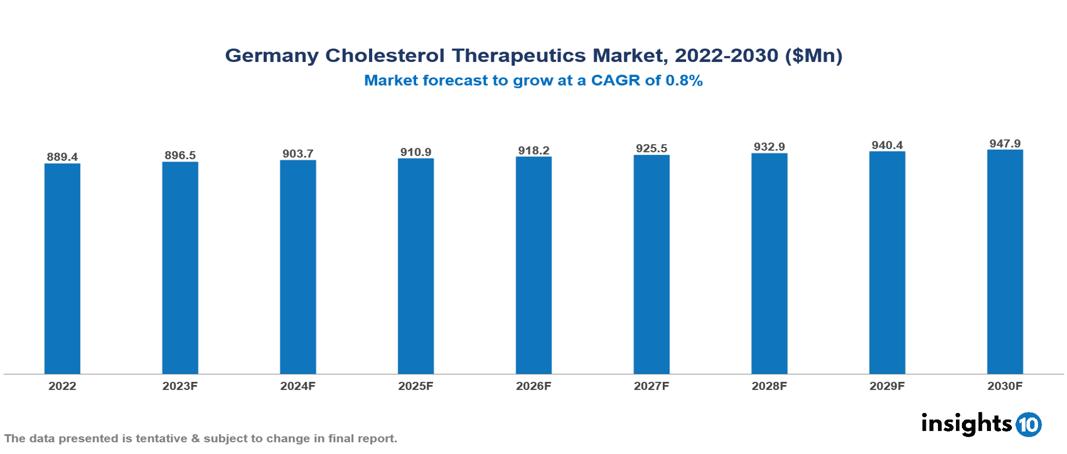
The Germany Cholesterol Therapeutics Market is anticipated to experience a growth from $889 Mn in 2022 to $948 Mn by 2030, with a CAGR of 0.8% during the forecast period of 2022-2030. The key drivers include the demographic shift towards an aging population with an increased risk of cardiovascular diseases, a simultaneous rise in healthcare, and the significant impact of strategic collaborations and partnerships fostering innovation and the development of novel therapies. The Germany Cholesterol Therapeutics Market encompasses various players across different segments Amgen, Pfizer, Bausch, AstraZeneca, Johnson & Johnson, Sanofi, Novartis, AbbVie, Dr Reddy’s Laboratories, Boehringer Ingelheim, etc., among various others.
Buy Now

Germany Cholesterol Therapeutics Market Analysis Executive Summary
The Germany Cholesterol Therapeutics Market is anticipated to experience a growth from $889 Mn in 2022 to $948 Mn by 2030, with a CAGR of 0.8 % during the forecast period of 2022-2030.
Cholesterol, a fatty substance essential for constructing cell membranes and hormones, plays a crucial role in bodily functions. However, imbalances can lead to health complications. Elevated levels of low-density lipoprotein (LDL) cholesterol, often referred to as "bad cholesterol," can contribute to atherosclerosis, elevating the risk of heart diseases and strokes. Conversely, high-density lipoprotein (HDL) cholesterol, known as "good cholesterol," aids in removing LDL from the bloodstream. Various conditions are linked to abnormal cholesterol levels, with atherosclerosis— the buildup of cholesterol in arterial walls—serving as a precursor to cardiovascular diseases. Elevated cholesterol levels also pose risks for conditions like coronary artery disease, peripheral artery disease, and stroke. Treatment strategies for cholesterol management focus on lowering LDL and increasing HDL. Lifestyle adjustments, such as adopting a heart-healthy diet, engaging in regular exercise, and managing weight, constitute the cornerstone. Medications, including statins that inhibit cholesterol production, and other drugs like bile acid sequestrants and PCSK9 inhibitors, may be prescribed to lower cholesterol. Ongoing research explores innovative therapies and personalized approaches to enhance the effectiveness of cholesterol management.
The prevalence of cholesterol-related disorders in Germany is a major issue, with high cardiovascular death rates and a relatively high frequency of excessive cholesterol levels. The reported prevalence of high cholesterol in Germany is 24.6%, greater than the OECD average of 18.0%. Cardiac heart disease is more prevalent in males than in women in Germany.
The key drivers include the demographic shift towards an aging population with an increased risk of cardiovascular diseases, a simultaneous rise in healthcare, and the significant impact of strategic collaborations and partnerships fostering innovation and the development of novel therapies.
Global companies like Merck and Pfizer are serious challengers for the top place in Germany. Both firms have a strong market presence and developed brand portfolios. Other major players include Novartis, Sanofi, and AstraZeneca, but with a lesser market share. The generic market contains several local enterprises that are actively developing.
Market Dynamics
Market Growth Drivers
Aging Population and Cardiovascular Risk: The demographic shift towards an aging population in Germany has significant implications for public health. As individuals age, the prevalence of cardiovascular diseases tends to rise. Given that hypercholesterolemia is a recognized risk factor for cardiovascular issues, the demand for cholesterol-lowering medications is likely to grow. This trend underscores the importance of targeted healthcare interventions for the elderly population to manage cholesterol levels and mitigate cardiovascular risks.
Increased Healthcare Expenditure and Access: A parallel trend driving the cholesterol therapeutics market in Germany is the overall increase in healthcare spending. A higher financial commitment to the healthcare sector can enhance access to essential services, including comprehensive cholesterol management and medication. This financial support facilitates a more robust infrastructure for preventive measures, early detection, and effective treatment of hypercholesterolemia, contributing to an overall improvement in public health outcomes.
Strategic Collaborations and Partnerships: The landscape of cholesterol therapeutics is significantly influenced by collaborations and partnerships among pharmaceutical companies, research institutions, and healthcare providers. These synergies often lead to the joint development of innovative therapies and treatment approaches. Collaborative efforts foster the exchange of expertise, resources, and research findings, potentially accelerating the discovery and introduction of novel cholesterol-lowering drugs. As a result, strategic partnerships play a pivotal role in driving market expansion and advancing the quality of available therapeutic options.
Market Restraints
Cost Containment Measures and Pricing Regulations: As an integral part of the European Union, Germany frequently employs stringent cost containment measures within its healthcare system. This involves the implementation of policies such as drug price controls and negotiations, aiming to regulate and reduce healthcare expenditures. While these measures contribute to controlling healthcare costs for consumers, they can pose challenges for pharmaceutical companies by limiting the pricing flexibility of cholesterol-lowering medications.
Low Awareness and Screening Deficiencies: A notable restraint on the growth of the cholesterol therapeutics market in Germany is the potential lack of awareness regarding the significance of cholesterol management and associated health risks. If public awareness remains insufficient, it may contribute to low screening rates for hypercholesterolemia. In turn, this can result in underdiagnosis and undertreatment of individuals with high cholesterol levels.
Healthcare Policies and Regulatory Landscape
The Federal Institute for Drugs and Medical Devices (BfArM) in Germany serves as a crucial regulatory body overseeing the safety and efficacy of medications and medical equipment, ensuring public health and safety. BfArM is responsible for evaluating and approving drugs, medical devices, and treatments, encompassing tasks such as quality control, post-marketing surveillance, licensing, and regulatory reviews. Through collaboration with both domestic and international partners, including pharmaceutical companies and health authorities, BfArM upholds rigorous standards, contributing to the preservation of medical product integrity, fostering healthcare innovation, and safeguarding the public from potential risks. As an integral component of Germany's healthcare system, BfArM plays a pivotal role in upholding the country's commitment to delivering safe and effective healthcare services to its residents.
Competitive Landscape
Key Players:
- Amgen
- Pfizer
- Bausch
- AstraZeneca
- Johnson & Johnson
- Sanofi
- Novartis
- AbbVie
- Dr Reddy’s Laboratories
- Boehringer Ingelheim
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Cholesterol Therapeutics Market Segmentation
By Indication
- Hypercholesterolemia
- Hyperlipidaemia
- Cardiovascular Diseases
- Others
By Drug Class
- Statins
- Bile Acid Sequestrants
- Lipoprotein Lipase Activators
- Fibrates
- Others
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By End User
- Hospitals
- Speciality Clinics
- Homecare
- Academics & Research Centers
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.