Germany Charcot-Marie-Tooth Disease (CMT) Therapeutics Market Analysis
Germany Charcot-Marie-Tooth Disease (CMT) Therapeutics market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. The majority of hereditary peripheral neuropathies are Charcot-Marie-Tooth (CMT) illness, commonly referred to as hereditary motor and sensory neuropathy (HMSN). Genes that support or generate proteins important in the construction and operation of the myelin sheath or the peripheral nerve axon are mutated, leading to CMT. An increase in genetic illnesses will result in more innovations being made in this area, which will enhance market growth because there are many genes that cause these problems. The above-mentioned forecast period will witness the emergence of the market for Charcot Marie-Tooth disease due to multiple prospects that will be heightened by the rising focus on targeted therapies and the favorable government regulations. Addex Therapeutics Ltd., Affectis Pharmaceuticals AG, Genzyme Corp., Lead Discovery Center GmbH, Pharnext SA, Acceleron Pharma, MedDay Pharmaceuticals, Bristol-Myers-Squibb Company, Inflectis Bio Science Health Company, Helixmith Co., Ltd., and Neurogene Inc. are some of the major players in the Charcot Marie Tooth Disease Market.
Buy Now

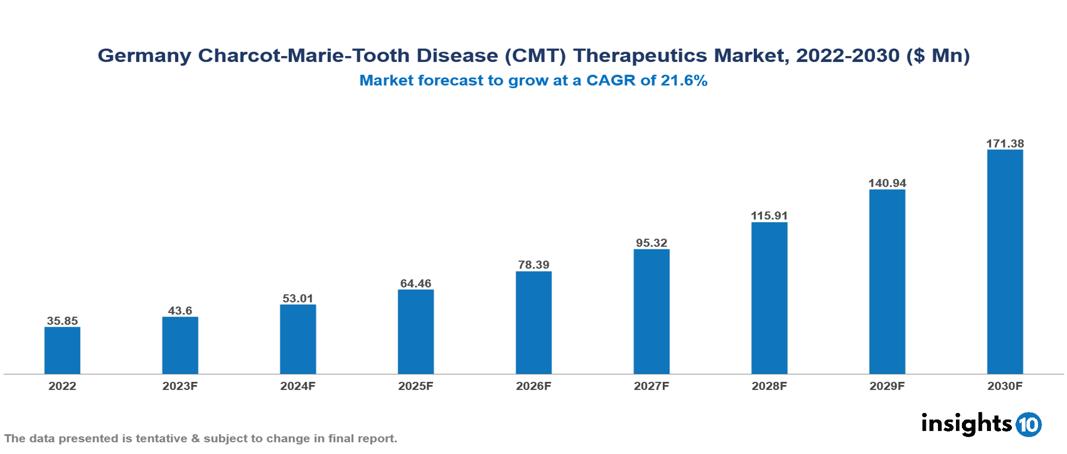
Germany Charcot-Marie-Tooth Disease (CMT) Therapeutics Market Analysis Summary
Germany Charcot-Marie-Tooth Disease (CMT) Therapeutics Market is valued at around $35.85 Mn in 2022 and is projected to reach $171.38 Mn by 2030, exhibiting a CAGR of 21.6% during the forecast period 2023-2030.
Peripheral nerves are harmed by CMT, a gradual, degenerative condition. More than 3 million people worldwide and 150 000 Americans are impacted by it. The peripheral nerves, which carry messages and impulses from the brain and spinal cord to and from the rest of the body as well as sensory information like touch, back to the brain and spinal cord, are damaged by a variety of conditions, including Charcot-Marie-Tooth disease (CMT). Several different genetic mutations can result in CMT. The nerves that govern the muscles can also be directly impacted by CMT. It is one of the most common rare diseases, yet there are no viable therapies or solutions for it at the moment. A little more than half of all CMT patients have the most common type, CMT1A. Though the condition can start at any age, progressive muscle weakness usually becomes apparent in teens or early adulthood.
The symptoms typically start in the feet and lower legs and can subsequently spread to the fingers, hands, and arms since longer nerves are afflicted first. Despite the fact that some CMT patients may never be aware of their condition, the majority of them experience some level of physical impairment. One of the most prevalent inherited neurological disorders, CMT, also known as hereditary motor and sensory neuropathy, affects an estimated 126,000 people in the United States and 2.6 million people globally.
It is almost always hereditary. When a person has mutations in two or more genes, each of which causes a specific form of CMT, this might result in the development of two or more distinct forms of the illness. The genetic makeup of CMT is heterogeneous, which means that distinct gene mutations may result in symptoms that are similar to one another.
Augmentation of genetic abnormalities & increase in developments in this area will spur market expansion because there are several genes that cause this disease. The above-mentioned forecast period will see the emergence of the Charcot-Marie-tooth disease market due to a number of factors, including the increased focus on targeted medicines and favourable government regulations.
Addex Therapeutics Ltd., Affectis Pharmaceuticals AG, Genzyme Corp., Lead Discovery Center GmbH, Pharnext SA, Acceleron Pharma, MedDay Pharmaceuticals, Bristol-Myers-Squibb Company, Inflectis Bio Science Health Company, Helixmith Co., Ltd., and Neurogene Inc. are some of the leading key players operating in the Charcot Marie Tooth Disease Market.
Market dynamics
Market Drivers
Growth of the market will be boosted by an increase in advances in this area due to the rise in genetic illnesses, which are caused by a high number of genes. The above-mentioned forecast period will see the emergence of the Charcot-Marie-tooth disease market due to a number of factors, including the increased focus on targeted medicines and favourable government regulations.
Market Developments
?The FDA has designated VCA-894A as an orphan drug for the treatment of Charcot-Marie-Tooth disease, axonal type 2S (CMT2S), which is brought on by cryptic splice site variations in IGHMBP2. This was reported by Vanda Pharmaceuticals Inc. today.
For the treatment of Charcot-Marie-Tooth disease type 1A (CMT1A), a rare, crippling, inherited peripheral neuropathy, Pharnext is developing PXT3003, a first-in-class therapeutic candidate.
The annual meeting of the Peripheral Nerve Society (PNS) in 2023 will focus on Charcot-Marie-Tooth (CMT) disease. Oryzon chose ORY-4001 as a candidate for clinical development after this partnership produced promising preclinical findings. This drug has a superb safety profile that prevents hematotoxicity thanks to its high selectivity against other HDAC classes and superior pharmacology as an HDAC6 inhibitor. The drug's in vivo studies in inflammatory models are encouraging, as it demonstrates potent anti-inflammatory capabilities.
Key players
BioMarin Pharmaceutical Pfizer Shire Genzyme Takeda Pharmaceutical Company Protalix BioTherapeutics Kedrion Biopharma Baxter International GlycoMimetics Alexion Pharmaceuticals1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market segmentations for Germany Charcot-Marie-Tooth Disease (CMT) Therapeutics market
By Disease Type
- CMT 1
- CMT 2
- CMT 3
- Others
By treatment
?By Drug Type
- Nonsteroidal anti-inflammatory drugs
- Cyclooxygenase-2 inhibitors
- Tricyclic antidepressants
- Anticonvulsants
- Analgesics
- Pipeline Drug
By surgery
- Soft-tissue procedures
- Osteotomy
- Joint-stabilizing procedures
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































