Germany Central Nervous System (CNS) Therapeutics Market Analysis
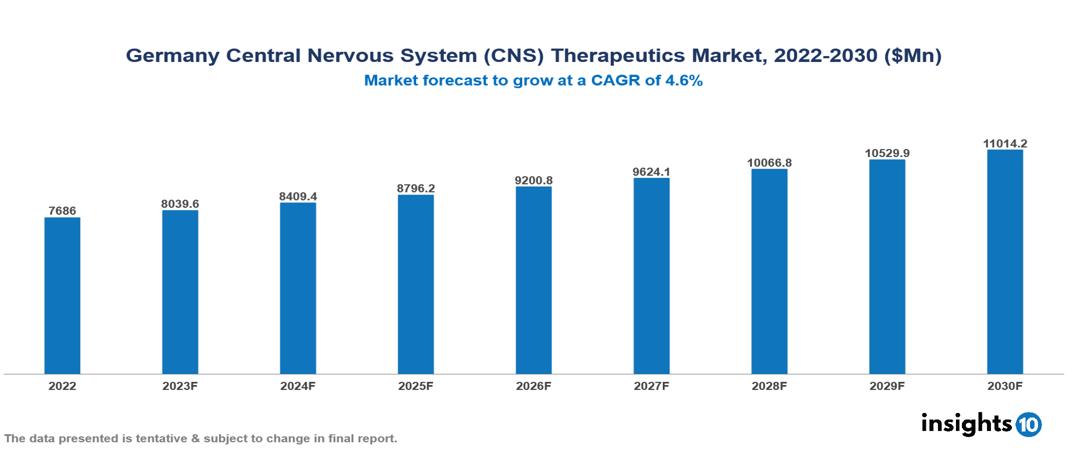
The Germany Central Nervous System (CNS) Therapeutics Market was valued at $7.686 Bn in 2022 and is predicted to grow at a CAGR of 4.6% from 2023 to 2030, to $11.014 Bn by 2030. The key drivers of this industry include the surge in the prevalence of CNS disorders, government initiatives, and technological advancements in drug discovery. The industry is primarily dominated by players such as AbbVie, Sandoz, Eli Lilly, Mylan, Ratiopharm, and Biogen among others.
Buy Now

Germany Central Nervous System (CNS) Therapeutics Market Analysis
The Germany Central Nervous System (CNS)Therapeutics Market is at around $7.686 Bn in 2022 and is projected to reach $11.014 Bn in 2030, exhibiting a CAGR of 4.6% during the forecast period.
Central Nervous System (CNS) disorders constitute a broad spectrum of medical conditions impacting the proper functioning of the brain and spinal cord, leading to disruptions in normal neurological processes. This category encompasses various disorders, including neurodegenerative conditions like Alzheimer's and Parkinson's, psychiatric disorders such as depression and schizophrenia, as well as neurological ailments like epilepsy and multiple sclerosis. The causes of CNS disorders are diverse, involving factors like genetic predisposition, environmental influences, infections, injuries, and autoimmune responses. While symptoms vary based on the specific disorder, they often manifest as disturbances in cognitive function, motor skills, mood, or sensory perception. Therapeutic approaches for CNS disorders aim to mitigate symptoms, slow disease progression, or address associated complications. Treatment modalities include medications, psychotherapy, and, in certain cases, surgical interventions. Prominent pharmaceutical companies actively involved in developing medications for CNS disorders include industry leaders like Pfizer, Eli Lilly, and Johnson & Johnson. For instance, Pfizer specializes in medications for Alzheimer's disease, while Eli Lilly is renowned for its advancements in psychiatric medications.
Germany experiences a significant burden of neurological disorders affecting more than 15% of individuals annually. The market therefore is propelled by important factors like the rising prevalence of neurological diseases, government initiatives, and technological advancements in drug discovery. However, limited private investment and collaborations, a stringent regulatory environment, and a lack of human resources limit the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Increasing burden of CNS diseases: The rising prevalence of age-related neurodegenerative diseases such as Alzheimer's and Parkinson's in Germany is driven by the aging demographic. The forecasted period anticipates a notable increase in the number of individuals affected by dementia. Germany also grapples with a significant occurrence of mental health disorders, where 9.2% of the population experiences major depressive disorder, and 15.4% is affected by anxiety disorders annually. The growing demand for treatment for these conditions contributes to the expansion of the market.
Supportive government initiatives: The German government places a high priority on healthcare, dedicating substantial resources to research, development, and the accessibility of innovative therapies for the CNS. Market expansion is further propelled by mental health initiatives focused on enhancing care accessibility, diminishing stigma, and encouraging early intervention.
Technological advancements in drug discovery: Germany possesses a strong research and development foundation in the life sciences field. Progress in fields such as genomics, personalized medicine, and digital therapeutics is driving the creation of more precise and efficient therapies for the central nervous system. An illustration of this is the integration of artificial intelligence (AI) in drug discovery, which expedites the identification of novel drug candidates and streamlines their development processes.
Market Restraints
Limited private investment and collaboration: Despite Germany's robust research infrastructure, the allocation of public and private funds for central nervous system (CNS) research is comparatively lower than in certain other developed nations. This discrepancy may pose a challenge to the advancement of innovative therapies designed to address specific needs. Inadequate collaboration among academic researchers, pharmaceutical firms, and government agencies has the potential to impede the seamless transfer of knowledge and the translation of research discoveries into practical clinical applications.
Stringent regulatory landscape: Securing approval for novel CNS drugs in Germany involves maneuvering through rigorous regulations and undergoing multi-phase assessments. This process can result in substantial delays, extending the time it takes for these treatments to reach the market by several years, consequently impeding patients' timely access to potentially transformative therapies. The expenses related to regulatory adherence, including detailed documentation, inspections, and post-approval surveillance, pose considerable financial burdens for companies. This scenario may discourage investment in the German market, particularly for smaller enterprises or those dedicated to pioneering therapeutic innovations.
Lack of human resources: Germany is confronted with an increasing deficit of neurologists, psychiatrists, and other mental health professionals, particularly in rural regions. This shortage hampers the ability to effectively address the escalating burden of central nervous system disorders.
Notable Updates
August 2023, New York City-based TG Therapeutics announced an agreement with Neuraxpharm Group valued at $645 Mn, providing the German specialty pharmaceutical company with exclusive marketing rights for Briumvi (ublituximab) beyond the borders of the United States.
March 2023, Secarna Pharmaceuticals GmbH & Co. KG, a biopharmaceutical firm dedicated to discovering and advancing next-generation antisense oligonucleotide (ASO) therapies for challenging or previously difficult-to-target subjects, has entered into a multi-target research and option agreement with SciNeuro Pharmaceuticals ("SciNeuro"), a leader in pioneering therapeutics for neurological disease treatment. This collaboration involves joint efforts to develop innovative ASO therapies targeting specific elements crucial to Central Nervous System diseases.
Healthcare Policies and Regulatory Landscape
The regulatory authority for therapeutics in Germany is the Federal Institute for Drugs and Medical Devices (BfArM) and the Paul Ehrlich Institute (PEI). BfArM is responsible for the approval and supervision of pharmaceuticals, while PEI focuses on biological medicinal products, including vaccines and immunotherapies. These regulatory bodies work in collaboration to ensure the safety, efficacy, and quality of therapeutic products in Germany.
To obtain licensure for therapeutics in Germany, pharmaceutical companies typically undergo a thorough regulatory process. The regulatory authorities assess the documentation to evaluate the safety and efficacy of the therapeutic product. If the product meets the required standards, marketing authorization is granted. The process is designed to adhere to European Medicines Agency (EMA) guidelines and European Union regulations.
For new entrants into the German therapeutic market, the regulatory environment can be robust and rigorous. Compliance with high-quality standards and adherence to regulatory guidelines is essential. The environment encourages innovation but requires thorough documentation and adherence to regulatory procedures.
Competitive Landscape
Key Players
- AbbVie
- Johnson & Johnson
- Eli Lilly
- Mylan
- Teva Pharmaceuticals
- Ratiopharm
- Biogen
- Sunovion
- Sandoz
- Pfizer
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Central Nervous System (CNS)Therapeutics Market Segmentation
By Drug
- Biologics
- Non-Biologics
By Drug Class
- Antidepressants
- Analgesics
- Immunomodulators
- Interferons
- Decarboxylase Inhibitors
- Others
By Disease
- Neurovascular Disease
- Degenerative Disease
- Infectious Disease
- Mental Health
- CNS Cancer
- Others
By Distribution Channel
- Hospital based pharmacies
- Retail pharmacies
- Online pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.