Germany Cardiovascular Drugs Market Analysis
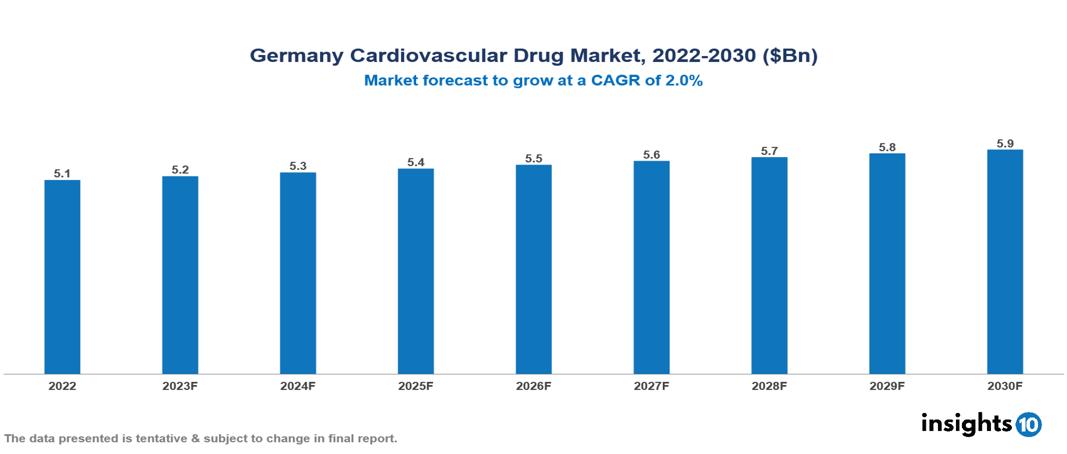
Germany Cardiovascular Drug Market is at around $5.06 Bn in 2022 and is projected to reach $5.93 Bn in 2030, exhibiting a CAGR of 2% during the forecast period. The incidence of cardiovascular diseases and increased public awareness of cardiovascular health drive market growth. The market is dominated by key players like Bristol-Myers Squibb Co, Pfizer, Bayer AG, Janssen Pharmaceuticals, Inc., AstraZeneca Inc., Sanofi-Aventis Inc., Novartis Pharmaceuticals, Merck & Co., Inc., Gilead Sciences, Inc., and F. Hoffman-La Roche Ltd.
Buy Now

Germany Cardiovascular Drug Market Executive Summary
Germany Cardiovascular Drug Market is at around $5.06 Bn in 2022 and is projected to reach $5.93 Bn in 2030, exhibiting a CAGR of 2% during the forecast period.
Heart attacks, strokes, and venous thromboembolism are classified as cardiovascular disorders since they affect the blood and lymphatic vessels in addition to the cardiovascular system. Heart attacks and strokes are usually acute events caused by a blockage that prevents blood flow to the heart or brain. The appropriate medications and technologies should be available to patients with cardiac problems. Aspirin, beta-blockers, angiotensin-converting enzyme inhibitors, and statins are examples of fundamental drugs that ought to be accessible to patients.
Germany's cardiovascular medication market is steadily expanding because of the use of tailored therapy. The aging population's growing knowledge of cardiovascular problems is fueling the market's expansion. Major companies must focus on R&D for innovative therapies and tactical alliances to maintain their market leadership due to the realities of the competitive landscape.
The pharmaceutical industry is responsible for drug discovery, production, distribution, and research. Over the previous two decades, pharmaceutical revenues rose rapidly, reaching $138.33 Bn worldwide in 2022. With the introduction of new technologies and more economical and effective manufacturing techniques, the pharmaceutical industry has undergone a significant transformation. Furthermore, the growing amount of capital flowing into this sector has helped to propel the market's expansion.
With a solid reputation for developing cutting-edge cardiovascular treatments, Bristol-Myers Squibb is a powerful brand. Eliquis makes a sizable profit from its leading position in the anticoagulant sector. With possible blockbuster medications filling gaps in the market, their pipeline looks bright. They also collaborate with top German institutes and actively participate in clinical research.
Market Dynamics
Market Growth Drivers:
Clinical Trials and Research: Ongoing cardiovascular clinical trials and research studies add to the pipeline of novel medications, which could eventually lead to a long-term expansion of the market.
Partnerships and Collaborations: Research centers, pharmaceutical corporations, and healthcare providers can work together to promote innovation and the creation of novel cardiovascular medications.
Education and Awareness: The need for therapeutic and preventive drugs may be fuelled by a greater public understanding of the dangers of cardiovascular illnesses and the value of early intervention.
Market Restraints:
Healthcare Reimbursement Policies: Modifications to reimbursement schedules and policies may have an impact on cardiovascular medication accessibility and affordability. Patient access to certain medications may be impacted by low reimbursement rates or coverage limitations.
Patent Expirations: Generic competition enters the market when branded cardiovascular medication patents expire. This may cause the original drug's market share to drop quickly and cause the pharmaceutical business to lose money.
Changes in Disease Patterns: Demand for particular cardiovascular medications may be impacted by changes in the prevalence or patterns of disease. For instance, there might be less of a market for medications that target a specific cardiovascular ailment if the condition's occurrence declines.
Healthcare Policies and Regulatory Landscape
The approval authority in Germany for pharmaceuticals is the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM). Clinical studies are used to demonstrate the safety and efficacy of novel pharmaceuticals. Before a product is released onto the market in Germany, clinical trials are carried out and approved by the relevant federal higher authority. Germany's lengthy examinations and strict regulatory standards make the drug approval procedure complicated. Complicated factors include the requirement for extensive clinical trial data and detailed evaluations of safety, efficacy, and quality.
Competitive Landscape
Key Players:
- Bristol-Myers Squibb Co.
- Pfizer
- Bayer AG
- Janssen Pharmaceuticals, Inc.
- AstraZeneca Inc.
- Sanofi-Aventis Inc.
- Novartis Pharmaceuticals
- Merck & Co., Inc.
- Gilead Sciences, Inc.
- F. Hoffmann-La Roche Ltd
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Cardiovascular Drug Market Segmentation
By Drug Type
- Cephalosporins
- Antihypertensive
- Antihyperlipidemic
- Anticoagulants
- Antiplatelet Drug
- Other
By Disease Indication
Hypertension
- Hyperlipidemia
- Coronary Artery Disease
- Arrhythmia
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































