Germany Brain Cancer Therapeutics Market
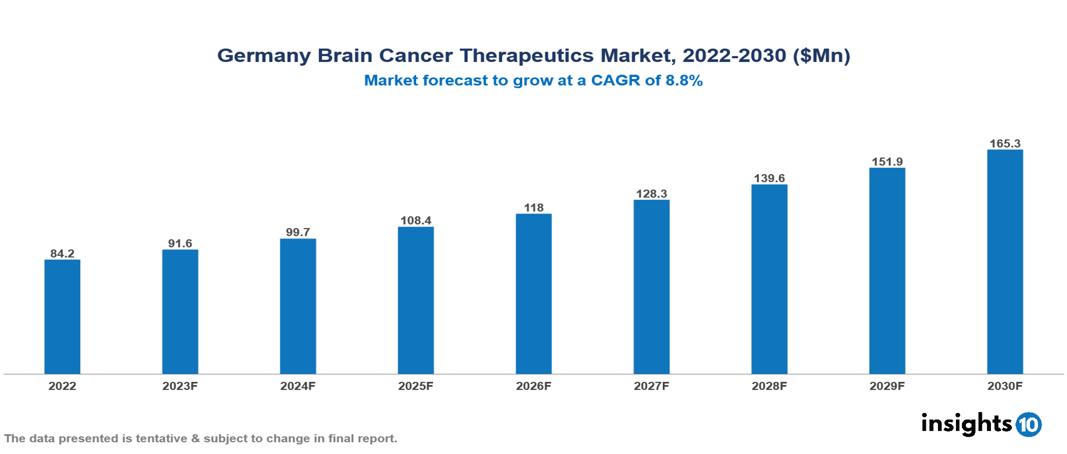
Germany Brain Cancer Therapeutics Market valued at $84 Mn in 2022, projected to reach $165 Mn by 2030 with a 9.8% CAGR. The expected rise in incidents of brain cancer, specifically glioblastoma multiforme, is projected to significantly drive the demand for brain cancer therapeutics, influencing the overall market scenario. Leading pharmaceutical companies currently working in the market are Roche, Merck & Co., Bristol Myers Squibb, Novartis, Pfizer, AstraZeneca, Eli Lilly, Sanofi, Takeda Pharmaceutical, and AbbVie.
Buy Now

Germany Brain Cancer Therapeutics Market Executive Summary
Germany Brain Cancer Therapeutics Market valued at $84 Mn in 2022, projected to reach $165 Mn by 2030 with a 9.8% CAGR.
The abnormal proliferation of brain cells serves as an indication of brain cancer, where these cells exhibit characteristics that can be either malignant (cancerous) or benign (non-cancerous). While the exact cause of brain cancer remains unclear, identified risk factors include exposure to ionizing radiation and a family history of brain tumors. Common symptoms associated with a brain tumor include headaches, gradual sensory decline, issues with balance, speech impediments, and hearing difficulties. The manifestation of these symptoms varies depending on factors such as the tumor’s size, location, and growth rate. Treatment approaches for brain cancer are determined by the tumor’s type, location, and size, with frequently utilized methods including radiosurgery, chemotherapy, radiotherapy, surgery, and the use of carmustine implants.
With 8,600 new cases of brain tumors diagnosed in Germany each year, the country has a 70.9% prevalence of brain cancer. In Germany, there was an apparent rise in the frequency of brain tumors up until roughly 2005. But by 2023, the incidence rates had leveled out and were about the same as those in the Nordic nations. In Germany, brain tumors accounted for 1.4% of all newly diagnosed cancer cases in 2023. It is noteworthy that the incidence rate of brain tumors is higher in men (5.1 per 100,000) than in women (3.6 per 100,000). Meningiomas, pituitary tumors, and gliomas, including glioblastoma multiforme, are the most common kinds of brain tumors in Germany.
CureVac AG, a biopharmaceutical company, is at the forefront of developing mRNA-based immunotherapy candidates for glioblastoma treatment. Their candidate, CVM-424, is dedicated to mRNA-based immunotherapy targeting EGFRvIII, a prevalent mutation in glioblastoma. Having entered Phase I clinical trials in December 2023, the initial data for CVM-424 is expected to be available in late 2024. Additionally, CureVac's pipeline includes CV9201, another mRNA candidate currently undergoing preclinical development. This therapy is intricately designed to target multiple glioma-associated antigens, exemplifying the company's dedication to addressing the complexities of glioblastoma treatment.
Market Dynamics
Market Growth Drivers
Increasing Incidence of Brain Cancer: The age-adjusted incidence rate of brain cancer in Germany stands at approximately 430 cases per 100,000 population, and there is an anticipated increase in this figure attributed to a growing elderly population. With the extension of life expectancy, the likelihood of developing cancer, including brain cancer, is on the rise.
Rising Demand for Personalized Medicine: A growing understanding of cancer genomes and tumor variety is fuelling interest in personalized medicine and providing the way to more specialized treatment approaches. With this strategy, specific mutations or pathways within patients' malignancies can be precisely targeted, potentially improving treatment outcomes. One of the main factors driving market expansion in Germany is the country's aggressive investment in customized medicine research and its application to clinical settings.
Increasing Awareness and Advocacy: Public awareness of brain cancer is on the rise, thanks to media campaigns and advocacy initiatives. This heightened awareness contributes to early detection and a growing demand for diverse treatment options. Advocacy groups are actively striving to enhance treatment accessibility and tackle issues impacting both patients and their families.
Market Restraints
Regulatory Hurdles: Stringent regulatory requirements and approval processes can pose challenges for the development and introduction of new brain cancer treatment drugs, potentially delaying their entry into the market.
High Development Costs: The research and development costs associated with creating effective brain cancer treatment drugs can be substantial. Companies may face financial constraints in funding extensive clinical trials and obtaining necessary approvals.
Limited Efficacy of Current Treatments: The efficacy of existing brain cancer treatments may be limited, and there could be a need for breakthrough innovations to significantly improve patient outcomes. This limitation may impact the market potential for current drugs.
Healthcare Policies and Regulatory Landscape
In Germany, healthcare policies and regulatory oversight for treatment drugs are primarily governed by the Federal Institute for Drugs and Medical Devices (BfArM) and the Paul-Ehrlich-Institute (PEI). BfArM, a federal agency within the Ministry of Health, is responsible for evaluating and approving drugs for the German market, ensuring their safety, efficacy, and quality. The PEI, another regulatory authority, focuses on advanced therapies and vaccines. The country adheres to the European Medicines Agency (EMA) regulations, contributing to a harmonized approach within the European Union. The Institute for Quality and Efficiency in Health Care (IQWiG) assesses the effectiveness and cost-effectiveness of pharmaceuticals. The healthcare policies in Germany aim to balance access to innovative treatments with cost containment, fostering a robust regulatory framework to safeguard public health while promoting medical advancements.
Competitive Landscape
Key Players
- Roche
- Merck & Co.
- Bristol Myers Squibb
- Novartis
- Pfizer
- AstraZeneca
- Eli Lilly
- Sanofi
- Takeda Pharmaceutical
- AbbVie
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Brain Cancer Therapeutics Market Segmentation
By Type
- Gliomas
- Meningiomas
- Pituitary Adenomas
- Vestibular Schwannomas
- Neuroectodermal Tumours
By Treatment
- Chemotherapy
- Immunotherapy
- Targeted Drug Therapy
- Radiation Therapy
- Others
By End-Users
- Hospitals
- Oncology Specialty Clinics
- Oncology Treatment Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































