Germany Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Analysis
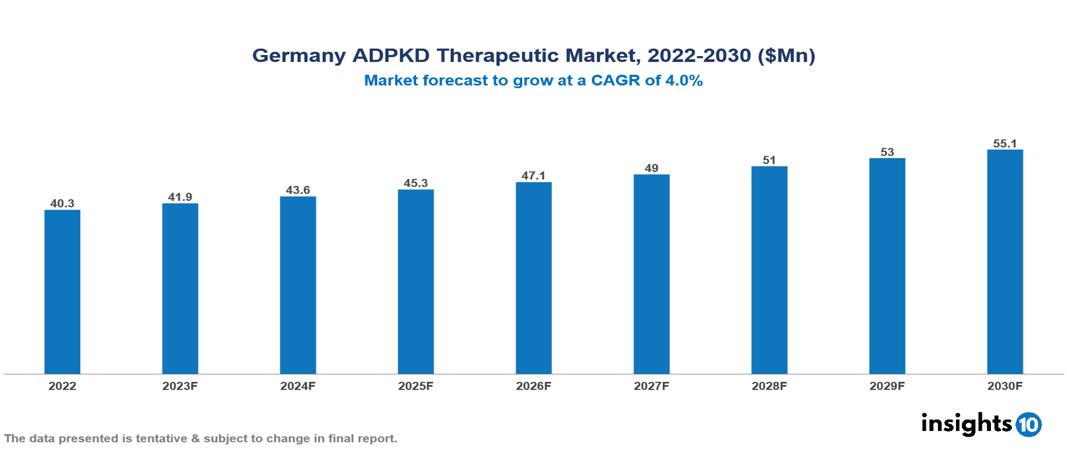
The Germany Autosomal Dominant Polycystic Kidney Disease Therapeutics Market was valued at US $ 40 Mn in 2022, and is predicted to grow at (CAGR) of 4% from 2023 to 2030, to US $55 Mn by 2030. The key drivers of this industry include the upward trend in the incidence of autosomal dominant polycystic kidney disease, strong healthcare infrastructure, and other factors. The industry is primarily dominated by players such as Otsuka, Sanofi, Roche, Biogen, Reata, Xortx, Janssen among others
Buy Now

Germany Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Analysis
The Germany Autosomal Dominant Polycystic Kidney Disease Therapeutics Market is at around US $ 40 Mn in 2022 and is projected to reach US $55 Mn in 2030, exhibiting a CAGR of 4% during the forecast period.
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic condition that affects multiple organs and is caused by mutations in the PKD1 and PKD2 genes. This leads to the development of fluid-filled cysts in the kidneys, causing them to enlarge (renomegaly). In most cases, ADPKD progresses to kidney failure (ESKD). Common symptoms include pain, frequent infections, and fatigue. Although there is no cure for this rare disease, treatment focuses on preventing complications and managing symptoms. Tolvaptan (Jinarc) manufactured by Otsuka Pharmaceuticals, is the only approved drug for ADPKD, acting as a vasopressin blocker to slow cyst growth in some patients. Other treatment options include pain management and lifestyle modifications. Several therapies have emerged to treat ADPKD, including vasopressin V2 receptor antagonists (Tolvaptan), mTOR inhibitors (Sirolimus, Everolimus), somatostatin analogues (Octreotide), and glucoceramide synthase inhibitors (Venglustat), among others. However, not all of these treatments have been successful in preventing the progression of the disease.
Germany is a country that is situated in Central Europe. The incidence of ADPKD is around 2.4/10,000 individuals in Germany. The anticipated increase in ADPKD prevalence in Germany can be attributed to improved diagnostic methods that reduce missed diagnoses, an evolving treatment landscape that allows rapid development of new therapeutics, and increased funding for curative and preventive research initiatives. The market is therefore driven by major factors like increased prevalence, an evolving treatment landscape, and strong healthcare infrastructure in the industry. However, conditions such as high costs of treatment, regulatory challenges, and others hinder the growth and potential of the market.
Market Dynamics
Market Growth Drivers
Surge in the prevalence of ADPKD: Estimates suggest the minimum prevalence of ADPKD is 2.4/10,000 individuals in Germany. Estimates indicate that currently 80,000 individuals are living with ADPKD in Germany. These estimates are anticipated to result in patients requiring treatment like dialysis, resulting in growth of the market.
Evolving treatment landscape: Novel drugs such as tolvaptan and potential gene therapy methods enhance disease management, attracting investment into the market. Furthermore, advancements in diagnostic resources, such as genetic testing, allow for earlier identification and prompt intervention, widening market accessibility and improving patient outcomes, resulting in market expansion.
Strong healthcare infrastructure: Germany’s robust healthcare system includes modern medical institutes and a proficient nephrology workforce, ensuring ADPKD patients have access to specialized care. Initiatives such as Social Health Insurance (SHI) provide health insurance coverage and financial support for advanced treatments for ADPKD.
Patient Advocacy: Deutsche PKD Gesellschaft (German PKD Society) and other active patient advocacy groups raise awareness and educate patients, allowing increased access to diagnosis, treatment, and research opportunities, which results in the expansion of the market.
Market Restraints
High costs of treatment: Treatments for ADPKD, such as Tolvaptans, can be costly, causing a considerable financial burden on both patients and the healthcare system. These out-of-pocket expenditures hinder treatment, especially for those with inadequate insurance coverage, and can result in the non-compliance of patients, distorting the growth of the market in Germany.
Regulatory challenges: The rigorous and prolonged drug approval procedures in Germany substantially postpone the availability of innovative ADPKD treatments for patients, potentially creating a gap between Germany and other advanced nations in terms of treatment accessibility and restricting the growth of the market.
Health system disparities: The inequitable distribution of specialized nephrologists and advanced diagnostic resources across various German regions results in uneven patient care and treatment options. These disparities may contribute to unequal patient outcomes and impede market expansion in underserved areas.
Healthcare Policies and Regulatory Landscape
Germany's healthcare policy and regulatory environment are managed by several crucial authorities and agencies. The Federal Institute for Drugs and Medical Devices (BfArM) and the Paul Ehrlich Institute (PEI) are the primary entities responsible for healthcare regulations and licensing in Germany. The BfArM is responsible for the approval and licensing of drugs, while the PEI manages the approval and licensing of biologics and vaccines. To obtain registration and marketing authorization for pharmaceuticals and medical devices, companies must comply with the BfArM and PEI requirements.
This process involves submitting technical and scientific data to validate the product's safety, quality, and effectiveness. Moreover, companies must designate a local importer or distributor for product liability, and some low-risk products might be exempt from the registration process. Both the public and private healthcare sectors in the country offer a spectrum of opportunities for companies engaged in the healthcare industry. The German healthcare system is divided into three main areas: outpatient care, inpatient care (the hospital sector), and rehabilitation facilities. The institutions responsible for running the healthcare system include associations and health insurers, regulatory bodies, the Federal Ministry of Health, patient organizations, and self-help groups. The healthcare system is based on four basic principles: compulsory insurance, funding through insurance premiums, self-governance, and solidarity. The Federal Ministry of Health is responsible for policy-making and developing laws and administrative guidelines for self-governing institutions. The Ministry of Health directs a number of institutions and agencies responsible for dealing with healthcare issues, such as the Federal Institute for Drugs and Medical Devices.
Competitive Landscape
Key Players
- Otsuka Pharmaceutical Co., Ltd
- Janssen Pharmaceuticals
- Reata Pharmaceuticals
- AbbVie Inc
- Exelixis
- Bayer AG
- Biogen Inc
- Palladio Biosciences
- Xortx Therapeutics
- Pano Therapeutics
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Autosomal Dominant Polycystic Kidney Disease Therapeutics Market Segmentation
By Treatment
- Pain & Inflammation Treatment
- Kidney Stone Treatment
- Urinary Tract Infection Treatment
- Kidney Failure Treatment
By Route of Administration
- Oral
- Parenteral
- Others
By End User
- Hospitals
- Speciality Clinics
- Surgical Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.