Germany Alopecia (Hair Loss) Therapeutics Market Analysis
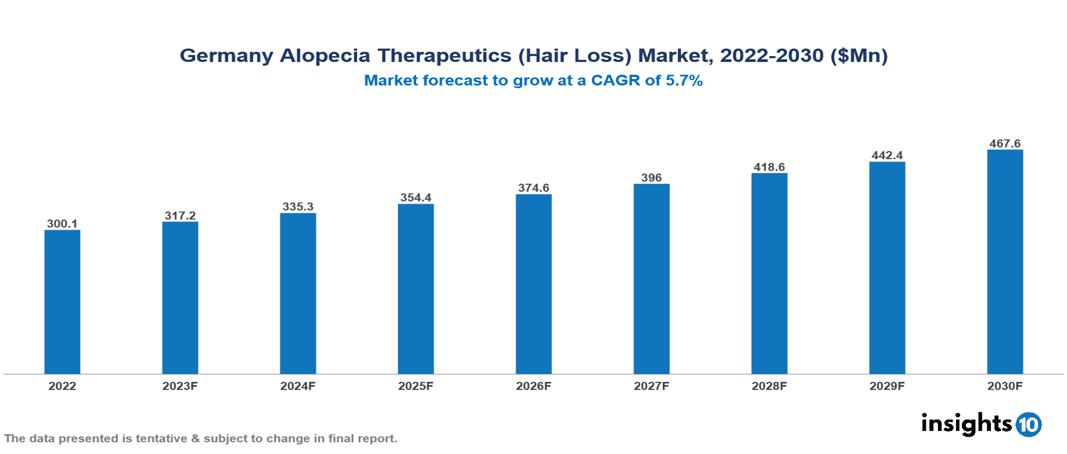
The Germany Alopecia (Hair Loss) Therapeutics Market was valued at US $300 Mn in 2022, and is predicted to grow at (CAGR) of 5.7% from 2023 to 2030, to US $468 Mn by 2030. The key drivers of this industry include the upward trend in the prevalence of alopecia (hair loss), supportive government initiatives, technological advancements, and others. The industry is primarily dominated by players such as, Eli Lilly, Merck, Pfizer, Bayer, Valeant Pharmaceuticals, and iRestore, among others.
Buy Now

Germany Alopecia Therapeutics Market Analysis: Executive Summary
The Germany Alopecia (Hair Loss) Therapeutics Market is at around US $300 Mn in 2022 and is projected to reach US $468 Mn in 2030, exhibiting a CAGR of 5.7% during the forecast period.
Alopecia, characterized by atypical hair loss, is an autoimmune hereditary disorder that affects a significant number of individuals. It presents in various forms, including localized or diffuse, temporary or permanent, and can impact individuals of all ages and genders. The condition is linked to multiple risk factors such as heightened stress levels, diabetes, inadequate nutrition, and environmental influences, resulting in notable distress among patients and influencing their overall quality of life. Common symptoms include hair loss, thinning hair, and bald patches on the scalp. Treatment options encompass applying topical medications like Minoxidil or corticosteroids directly to the scalp, as well as using injections and oral medications with more potent immunosuppressive effects. Light therapy and hair transplantation are also feasible alternatives. Companies such as Eli Lilly, with their JAK inhibitor Baricitinib, and Bayer, with Rogaine (minoxidil), are actively leading advancements in treatments for this condition.
The overall prevalence of alopecia is around 210/1,00,000 individuals in Germany. The increased prevalence of alopecia in Germany is caused by risk factors like chronic conditions like diabetes, environmental changes, and poor nutrition. The market is being fuelled by crucial factors such as the growing aging population and the increasing prevalence of alopecia, supportive government policies, and technological advancements in the therapeutics industry. However, challenges such as high costs of treatments like gene therapy, limited coverage, and limited market accessibility are a few factors that limit the market's potential.
Market Dynamics
Market Growth Drivers
Surge in prevalence of Alopecia: The overall prevalence of alopecia is estimated at 210/1,00,000 individuals, and the incidence is around 72/1,00,000 individuals in Germany. Around 41.2% of men in Germany undergo hair loss by the age of about 43 years, indicating the substantial demand for treatments that are effective. These estimates create a considerable pool of patients requiring advanced treatments, driving the market.
Government initiatives: Germany's robust healthcare system, coupled with extensive insurance coverage (social health insurance), creates a conducive environment for market expansion. Although certain treatments, such as JAK inhibitors, face challenges, government initiatives are advocating for enhanced reimbursement options, thereby expanding potential patient access.
Technological advancements: Advancements in hair analysis technology and diagnostic tools contribute to an improved understanding of alopecia subtypes and personalized treatment strategies, resulting in more effective care. The expansion of telemedicine and online consultations enhances access to specialists and treatment guidance, particularly in rural areas of Germany.
Advanced treatment options: The approval of Baricitinib (Olumiant) and the expected authorization of Rinvoq introduce novel oral medication alternatives that have the potential for enhanced efficacy and improved convenience compared to older treatments. Ongoing research in the evolving pipeline is dedicated to innovative therapies targeting different pathways related to alopecia, offering prospects for even more effective and personalized treatment options in the future.
Market Restraints
High costs of medications: JAK inhibitors such as Xeljanz and Baricitinib frequently exceed the standard limits of insurance coverage, resulting in substantial expenses for many patients. The financial strain is exacerbated by the costs of topical treatments and surgical procedures, creating difficulties for individuals with limited incomes to afford these therapies.
Limited coverage: While insurance plans might encompass certain alopecia treatments, particular medications or procedures like hair transplantation may be excluded or entail significant co-payments. This affects affordability and can shape decisions regarding care. Consequently, patients may bear the burden of co-payments, resulting in non-compliance with treatment and constraining market growth.
Limited accessibility: Limited access to specialists and specialized medications in rural areas results in geographic disparities in healthcare delivery in Germany. Despite having insurance coverage, some patients may still face restrictions on the number of treatment sessions or specific procedures, limiting their access to care.
Notable Updates
July 2023, Baricitinib (Olumiant) by Eli Lilly was approved in Germany. This JAK inhibitor provides a promising oral treatment alternative for individuals with moderate-to-severe alopecia areata. This marks a noteworthy change, as JAK inhibitors were not previously accessible for treating alopecia in Germany.
Healthcare Policies and Regulatory Landscape
Germany's healthcare policy and regulatory landscape are overseen by several pivotal authorities and agencies. The Federal Institute for Drugs and Medical Devices (BfArM) is the primary entity tasked with healthcare regulations and licensing in Germany. BfArM handles drug approval and licensing. To secure registration and marketing authorization for pharmaceuticals and medical devices, companies must adhere to the requirements set by BfArM.
This process involves submitting technical and scientific data to validate the product's safety, quality, and effectiveness. Additionally, companies must appoint a local importer or distributor for product liability, and certain low-risk products may be exempt from the registration process. The healthcare industry in Germany provides a spectrum of opportunities for companies, encompassing both the public and private sectors. The German healthcare system is divided into three main areas: outpatient care, inpatient care (the hospital sector), and rehabilitation facilities. Managing the healthcare system involves various entities, including associations, health insurers, regulatory bodies, the Federal Ministry of Health, patient organizations, and self-help groups.
The Federal Ministry of Health plays a crucial role in policymaking and developing laws and administrative guidelines for self-governing institutions.
Competitive Landscape
Key Players
- Eli Lilly
- Johnson & Johnson
- Pfizer
- Merck & Co
- Bayer
- Valeant Pharmaceuticals
- GlaxoSmithKline plc
- Aurobindo Pharma
- iRestore Hair Growth system
- Theradome
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Germany Alopecia Therapeutics Market Segmentation
By Disease Type
- Alopecia Areata
- Cicatricial Alopecia
- Traction Alopecia
- Alopecia Totalis
- Androgenetic Alopecia
- Alopecia Universalis
- Others
By Treatment Type
- Pharmaceuticals
- Devices
- Others
By Gender
- Male
- Female
By Route of Administration
- Topical
- Injectable
- Oral
By Age Group
- Below 18 years
- 18-34 years
- 35-49 years
- 50 years and above
By End User
- Hospitals
- Physician’s Office
- Dermatology clinics
- Others
By Sales Channel
- Prescriptions
- OTC
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.