France Periodontal Therapeutics Market Analysis
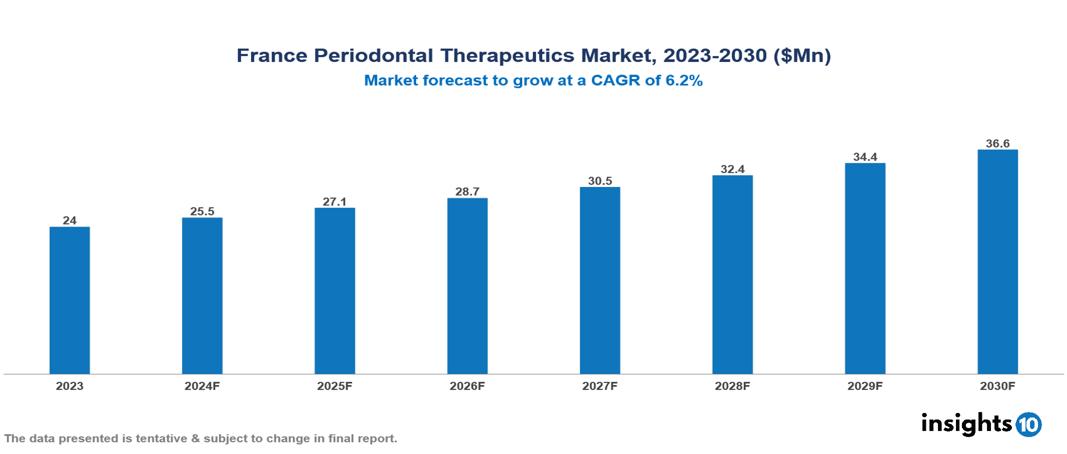
The France Periodontal Therapeutics Market was valued at $24 Mn in 2023 and is predicted to grow at a CAGR of 6.2% from 2023 to 2030, to $36.6 Mn by 2030. France Periodontal Therapeutics Market is growing due to Technological Developments, Rise in Demand for Non-surgical Periodontal Therapies, and Prevalence of periodontitis. The market is primarily dominated by players such as Pfizer Inc., Lupin Ltd, Teva Pharmaceuticals USA, Sun Pharmaceutical Industries Ltd, Tokyo Chemical Industry Co. Ltd., and Bausch Health Companies Inc.
Buy Now

France Periodontal Therapeutics Market Executive Summary
France Periodontal Therapeutics Market is at around $24 Mn in 2023 and is projected to reach $36.6 Mn in 2030, exhibiting a CAGR of 6.2% during the forecast period.
Gum disease, also known as periodontal disease, is a dangerous dental ailment associated with gum inflammation and infection. It surrounds the teeth. Periodontitis is a common disease that is usually caused by poor dental hygiene and can be mostly prevented with therapeutic, non-surgical, or surgical treatment options. The use of antibiotics alone to treat periodontitis is known as periodontal therapy, a relatively new yet successful treatment strategy that is gaining popularity among patients and dentists. Antibiotics like metronidazole, amoxicillin, and doxycycline are frequently used in periodontal therapy. Adjunctive therapies for reducing gingival inflammation and plaque include a variety of mouthwashes and rinses containing antimicrobial substances such hydrogen peroxide, chlorhexidine, and essential oils.
The periodontal market in France is driven by a prevalence of periodontal diseases affecting around 50% of the population, with severe cases in 10-15%. Demographic factors such as an aging population and increasing awareness of oral health contribute significantly to market growth. Healthcare expenditure on dental care in France is substantial, with a considerable portion allocated to periodontal treatments. The periodontal therapeutics market in France is also influenced by changes in lifestyle factors such as diet, smoking habits, and stress levels that can impact oral health and increase the risk of periodontal diseases. Public and private sectors invest in advanced technologies and preventive care, reflecting the country's commitment to addressing periodontal health issues. This market is expected to expand due to ongoing innovations and a focus on comprehensive dental care. Therefore, the market is driven by significant factors like Technological Developments, Rise in Demand for Non-surgical Periodontal Therapies, and Prevalence of periodontitis. However, Regulatory Complications, Side Effects, and Cost of treatment restrict the growth and potential of the market.
AMD Lasers announced the development of the Monet™ laser curing light, the first handheld laser in curing light for dental materials.
Market Dynamics
Market Growth Drivers
Technological Developments: Investing in dental technology yields significant economic benefits for periodontal practices. Digital workflows reduce chair time by up to 30%, increasing patient throughput. 3D imaging minimizes costly treatment revisions, improving first-time success rates. CAD/CAM systems cut lab fees by enabling in-house production of prosthetics. While initial investment in technology is substantial, practices report ROI within 2-3 years through increased efficiency and expanded service offerings.
Rise in Demand for Non-surgical Periodontal Therapies: Dentists are now offering more gentle ways to treat gum disease. Instead of surgery, they can often use deep cleaning methods like scaling and root planing. For tougher cases, they might add antibiotics to help fight the infection. These treatments are easier on you and often work just as well as surgery. New medicines are being developed that could make treatment even easier in the future. This means you might be able to get your gum disease under control with less discomfort and fewer visits to the dentist.
Prevalence of periodontitis: Periodontal disease can cause serious health concerns, including tooth loss, if left untreated. In order to prevent chronic inflammation, which raises the risk of diabetes, insulin resistance, and poor glucose tolerance, early therapy is essential. 50% of the population still has periodontal disease, despite the availability of therapies. Urbanization and shifting living conditions exacerbate this predominance and lead to an increase in oral health issues.
Market Restraints
Regulatory Complications: Strict reimbursement policies and price controls for dental procedures limit profitability and investment in advanced periodontal services. Regulatory requirements for clinical trials and product approvals further slow innovation adoption, constraining market expansion.
Side Effects: Increased worries about the aesthetic results of procedures like dental implants and periodontal plastic surgery provide problems for the periodontal industry. Patients place a higher priority on cosmetic outcomes than on functional restoration, with treatment acceptance and patient satisfaction being impacted by possible adverse effects including uneven gum lines or implant failure. As a result, healthcare professionals prioritize methods and goods that improve visual results without sacrificing usefulness.
Cost of treatment: Despite a robust public healthcare system, advanced dental procedures often require private sector involvement, leading to substantial out-of-pocket expenses. For example, 70% of specialized treatments, such as dental implants and regenerative surgeries, are financed privately, making them prohibitive for many citizens. Additionally, the stringent regulatory environment and high taxation on medical devices contribute to elevated prices. These financial constraints discourage patients from seeking timely periodontal care, limiting market expansion and the adoption of innovative treatments in the country.
Regulatory Landscape and Reimbursement scenario
Periodontics market is heavily influenced by regulatory frameworks that prioritize patient safety and treatment efficacy. Regulatory bodies like the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) oversee the approval and monitoring of periodontal products and treatments. Compliance with stringent standards ensures that products meet safety and quality requirements before entering the market. This regulatory environment shapes innovation and market dynamics, fostering a landscape where evidence-based practices and technological advancements in periodontics can thrive under careful scrutiny and approval processes.
Reimbursement plays a crucial role in patient access and treatment choices. The reimbursement system typically covers a significant portion of periodontal procedures, encouraging patients to seek necessary dental care without significant financial burden. This framework supports periodontists in providing comprehensive treatments, including scaling, root planning, and surgical interventions, ensuring optimal oral health outcomes. Additionally, adherence to reimbursement guidelines promotes transparency and equity in healthcare delivery, fostering patient trust and satisfaction within the French dental care system. Overall, reimbursement policies in France's periodontal market are pivotal in promoting accessible and effective periodontal care across the population.
Competitive Landscape
Key Players
Here are some of the major key players in France Periodontal Therapeutics Market:
- Pfizer Inc.
- Lupin Ltd
- Teva Pharmaceuticals USA, Inc.
- Sun Pharmaceutical Industries Ltd.
- Tokyo Chemical Industry Co., Ltd.
- Bausch Health Companies Inc.
- Melinta Therapeutics LLC
- Cipla, Inc.
- Chartwell Pharmaceuticals LLC.
- ASA Dental S.p.A.
- Steris-Hu-Friedy
- Carl Martin GmBH
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Periodontal Therapeutics Market Segmentation
By Disease
- Gingivitis
- Chronic Periodontal Disease
- Aggressive Periodontal Disease
- Others
By Drug Type
- Doxycycline
- Minocycline
- Chlorhexidine
- Metronidazole
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Channel
By Treatment procedures
- Scaling And Root Planing
- Gum Grafting
- Regenerative Therapy
- Dental Crown Lengthening
- Periodontal Pocket Procedures
- Single Tooth Dental Implants
- Multiple Tooth Dental Implants
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































