France Patient Adherence Programs Market Analysis
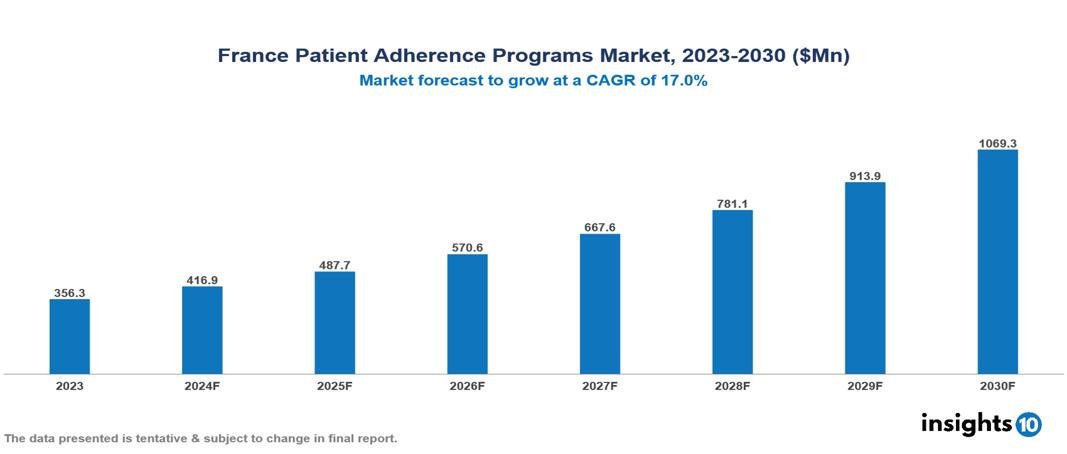
The France Patient Adherence Programs Market was valued at $356.3 Mn in 2023 and is predicted to grow at a CAGR of 17% from 2023 to 2030, to $1,069.3 Mn by 2030. The key drivers of the market include increasing non-adherence, rising chronic conditions, and an aging population. The prominent players in the France Patient Adherence Programs Market are Takeda, Pfizer, Sanofi, Novartis among others
Buy Now

France Patient Adherence Programs Market Executive Summary
The France Patient Adherence Programs market is at around $356.3 Mn in 2023 and is projected to reach $1,069.3 Mn in 2030, exhibiting a CAGR of 17% during the forecast period.
A patient adherence program aims to ensure that patients follow prescribed medication regimens to improve treatment outcomes, using both direct and indirect methods to assess adherence. Direct methods include monitoring therapy by measuring drug levels, metabolites, or biological markers in blood or urine, and confirming medication intake. Indirect methods, more commonly used, involve patient self-reports, pill counts, prescription refill rates, clinical response evaluations, and electronic medication monitors. Pill counts compare the number of pills taken between appointments with the prescribed dosage, while patient self-reports collect information through interviews, questionnaires, or diaries. Electronic devices such as pill bottles or blister packs track medication access to provide precise data. A widely used tool for assessing adherence is the Morisky Medication Adherence Scale (MMAS), a validated and reliable questionnaire suitable for clinical use. These approaches help healthcare providers ensure consistent medication use, ultimately enhancing patient health outcomes.
The burden of several chronic illnesses is significant, rising, and concerning among the older adult population. The France Patient Adherence Program Market is thus driven by significant factors such as increasing non-adherence, technological innovations, and healthcare cost containment. However, data privacy and security concerns, regulatory compliance, and patient-related challenges restrict the growth and potential of the market.
The major players in the France Patient Adherence Programs Market are Takeda, Sanofi, and Pfizer, all of which play pivotal roles in developing and implementing strategies to improve patient compliance and health outcomes through innovative adherence solutions.
Market Dynamics
Market Growth Drivers
Increasing Non-Adherence: The rising prevalence of medication non-adherence underscores the critical need for patient adherence programs. In France alone, 20% of patients fail to purchase prescribed medications, contributing to significant health consequences. Non-adherence is responsible for approximately 8,000 deaths annually and results in 1.1 million hospital days. These alarming statistics serve as key drivers for the growth of the patient adherence program market, highlighting the urgent demand for solutions that improve patient compliance and health outcomes.
Technological Innovations: Technologies like mobile health apps, wearables, telemedicine, and AI-driven reminders enable real-time tracking of medication intake, personalized interventions, and direct communication between patients and providers. These innovations help identify adherence barriers, improve patient engagement, and enhance health outcomes. By leveraging data analytics and machine learning to predict non-adherence risks, these tools drive the growth of the patient adherence program market, improving the efficiency, effectiveness, and scalability of adherence solutions while reducing healthcare costs.
Healthcare Cost Containment: The significant financial burden of medication non-adherence, estimated at $21 billion annually, is a major driver for the growth of patient adherence programs. These programs are increasingly recognized as essential for healthcare cost containment, as improving patient compliance can reduce unnecessary hospitalizations, medical interventions, and long-term healthcare expenses. By addressing non-adherence, these initiatives help lower overall healthcare costs and enhance patient outcomes, further fueling demand for effective adherence solutions.
Market Restraints
Data Privacy and Security Concerns: Data privacy and security concerns limit the adoption of patient adherence programs, as digital tools like health apps and wearables collect sensitive patient data, raising the risk of breaches. Fears of data misuse and strict compliance requirements, such as GDPR and HIPAA, can reduce patient trust and increase implementation costs, hindering the growth of these programs.
Patient-Related Challenges: Patient-related challenges significantly impact medication adherence programs in France. Barriers such as forgetfulness, confusion over medication instructions, side effects, and limited health literacy make adherence difficult for many. Socioeconomic factors, like financial constraints and unequal access to healthcare in rural areas, further exacerbate non-adherence. Psychological factors, including lack of motivation or denial of illness, also hinder compliance. Addressing these issues is crucial for the success of adherence programs across the French population.
Regulatory Landscape and Reimbursement Scenario
The main regulatory body for pharmaceuticals in France is the National Agency for the Safety of Medicines and Health Products (ANSM, Agence nationale de sécurité du médicament et des produits de santé in French). The ANSM is a government organization under the Ministry of Health that ensures the security of health products and facilitates access to innovative therapeutics. The ANSM guarantees the safety, efficacy, accessibility, and appropriate usage of health goods that are sold in France through evaluation, knowledge, and monitoring protocols.
The pharmaceutical companies must submit a completed MAA (Marketing Authorization Application) which can be used for drugs meant for the French market (National Procedure) or for the drugs intended for commercialization throughout the European Union (EU) through the EMA (European Medicines Agency). Through the EMA, products can be authorized through the National Procedure, the Centralised Procedure (CP), the Decentralised Procedure (DCP), or the Mutual Recognition Procedure (MRP). In this case, ANSM acts as a national competent authority (NCA) within the EMA framework. The ANSM then issues a final decision of either approval, conditional approval, or refusal after conducting a review and evaluation of the MAA based on safety, efficacy, quality, and risk-benefit ratio. The Medical Device and Technology Evaluation Committee (CNEDiMTS) is the agency that examines medical devices and classifies them based on their level of risk into the following four classes: Class I for low degree of risk, Class IIa for moderate degree of risk, Class IIa for high potential of risk, and Class III for very serious potential of risk.
The National Union of Health Insurance Funds of France (UNCAM, Union Nationale des Caisses d'Assurance Maladie) is the organization that is responsible for France’s public health insurance system. The UNCAM manages several social security health insurance funds in charge of reimbursing healthcare costs for French nationals and residents. The French Health High Authority evaluates a medicine’s Medical Benefit (Service Médical Rendu, SMR) considering the severity of the disease, safety and clinical efficacy, and therapeutic innovation. The Committee on Economic Products for Health (CEPS) negotiates with pharmaceutical firms on the cost of medications. Lastly, the Ministry of Health, based on the recommendations of HAS and CEPS, renders the ultimate decision about reimbursement.
Competitive Landscape
Key Players
Here are some of the major key players in the France Patient Adherence Programs Market:
- Takeda
- Sanofi
- Pfizer
- Novartis
- Astrazeneca
- Boehringer Ingelheim
- Amgen
- Adhere health
- Allazo Health
- Enliven Health
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Patient Adherence Programs Market Segmentation
By Type
- Hardware centric
- Software centric
By Medication
- Cardiovascular
- Nervous System
- Diabetes
- Gastrointestinal
- Oncology
- Rheumatology
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































