France Organ-On-Chip (OOC) Market Analysis
France Organ-On-Chip Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Organ-On-Chip is expanding as a result of increased demand of Organ-On-Chip (OOC) devices. This is fueling the growth of the organ-On-Chip market. Some of the key players in the global Organ-On-Chip Market include FzioMed, Inc., Amniox Medical, Inc., TissUse GmbH, AxoSim, BiomimX S.R.L., Elveflow, Emulate, Inc., H?rel Corporation, InSphero, MIMETAS, NORTIS, INC., and TARA Biosystems, Inc.
Buy Now

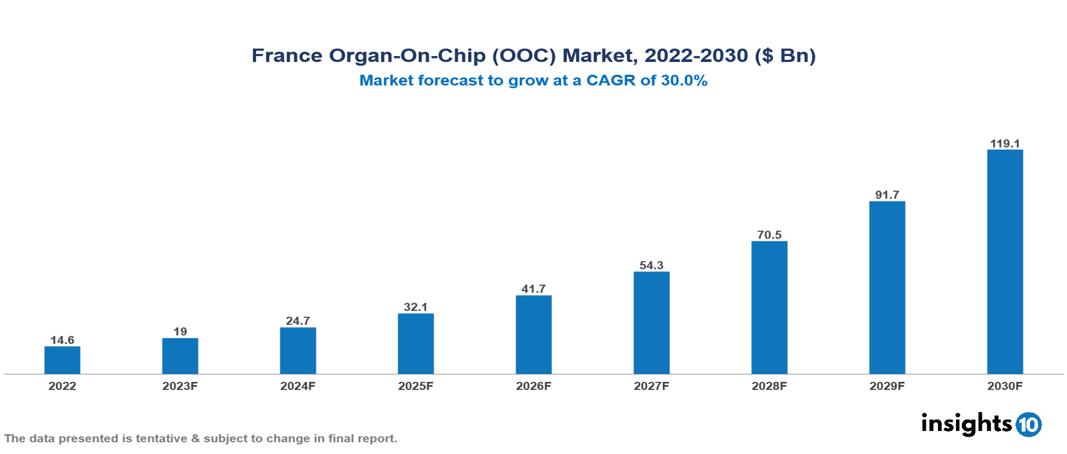
France Organ-On-Chip (OOC) Market Executive Summary
France Organ-On-Chip (OOC) Market is valued at around $14.6 Bn in 2022 and is projected to reach $118.9 Bn by 2030, exhibiting a CAGR of 30% during the forecast period 2023-2030.
Organ-on-chip is a multi-channel 3-D microfluid cell culture ship, stimulating activities and physiological response of entire organs, are being used to develop human organs on chips, including the lung, stomach, liver, heart, skin, bone marrow, pancreas, kidney, and even a system that replicates the blood-brain barrier.
According to the WHO's Global Tuberculosis Report 2022, 10.6 Mn people worldwide contracted tuberculosis (TB) in 2021, translating to 134 cases per 100,000 people. Also, the anticipated incidence rate of TB is projected to increase by 3.6% between 2020 and 2021. As a result, the prevalence of pulmonary diseases like tuberculosis is increasing, which raises the need for the management of disease utilizing Organ-On-Chip technology and propels the market.
Firms like FzioMed, Inc., Amniox Medical, Inc., TissUse GmbH, AxoSim, BiomimX S.R.L., Elveflow, Emulate, Inc., Hµrel Corporation, InSphero, MIMETAS, NORTIS, INC., and TARA Biosystems, Inc., are some of the major players in the Organ-On-Chip Market.
Real-time imaging, in-vitro biochemical analysis, and the metabolic and genetic activity of living cells in functioning tissue are the main applications of organ-on-chip technologies. The demand for organs on chips is anticipated to be driven by Organ-On-Chip’s constantly rising application breadth. The rising frequency of cardiovascular diseases (CVD) is also expected to take the market for Organ-On-Chip to new heights.
The increase in research activities is projected to fuel the market for Organ-On-Chip in the years to come. The market may yet have difficulties with regard to obtaining regulatory permission, as well as the high price of various Organ-On-Chip devices.
Market Dynamics
Drivers of France Organ-On-Chip Market:
Growing Demand for Organ-On-Chip (OOC) Devices: The increased demand for organ-on-chip technologies in the healthcare sector and the growing number of clinical studies and driving the growth of the market.
Increasing Research Activities: The demand for alternatives to animal testing, the need for the initial identification of treatment toxicity, the introduction of novel products, and technological advancements all contribute to the growth of the Organ-On-Chip market.
Support from the Government: Funding from the government to research institutes for regenerative medicine will boost the growth of the market.
Developments in Organ-On-Chip Market:
In 2022, Emulate Inc., in vitro model provider, announced its new application of adeno-associated virus transduction for a Liver chip that helps researchers test the safety and efficacy of adeno-associated virus vectors in a model of the liver that is relevant to humans with results in few weeks. This helps researchers to optimize gene therapy deliveries quickly and accelerates the development.
In 2022, Valo Health acquired TARA Biosystems, makers of a 3D heart tissue modeling platform. Valo planned to incorporate TARA’s heart tissue chips into an end-to-end drug development offering aimed at cardiovascular disease, driven by its Opal data platform.
Key players
Emulate, Inc. (French subsidiary) Poietis Axiogenesis AG (French subsidiary) Opticell4D Amicell Stilla Technologies Cellink France SAS ABL SA Gattefoss? SAS Prellis Biologics1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For France Organ-On-Chip (OOC) Market
By Organ Type
- Lung-On-Chip
- Heart-On-Chip
- Liver-On-Chip
- Kidney-On-Chip
- Intestine-On-Chip
- Skin-On-Chip
- Blood-Brain-Barrier-On-Chip
By Application
- Toxicology Research
- Drug Discovery
- Molecular Biology
- Disease Modelling
- Food Safety
By Material
- Polymers
- Glass
- Silicon
By End-user
- Biopharmaceutical Companies
- Academics
- Food & Beverages
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































