France Infectious Disease Diagnostic Market Analysis
France Infectious Disease Diagnostics Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for Infectious disease diagnostics is expanding as a result of rising prevalence of infectious diseases. This demand is driving the market for better diagnostics services for infectious diseases and methods for the early detection and accurate diagnosis of infectious disease. Some of the key players in the global Infectious Disease Diagnostics Market include Thermo-Fisher Scientific Inc., Hologic, Inc., Abbott, Bio-Rad Laboratories, Inc., OraSure Technologies, Inc, F. Hoffmann-La Roche Ltd., Siemens Healthineers, Chembio Diagnostics, Inc., Danaher Corporation, and Becton, Dickinson and Company.
Buy Now

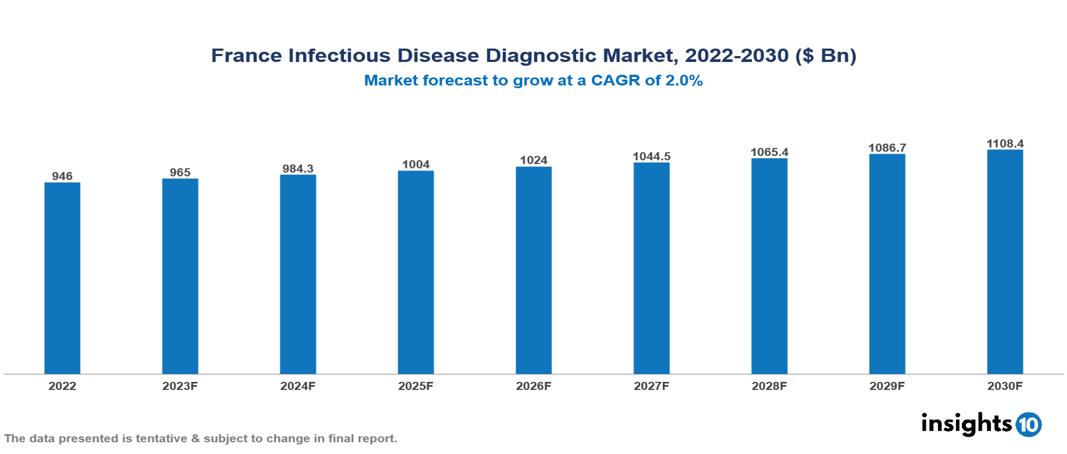
France Infectious Disease Diagnostic Market Executive Summary
France Infectious Disease Diagnostic Market is valued at around $946 Bn in 2022 and is projected to reach $1108.4 Bn by 2030, exhibiting a CAGR of 2% during the forecast period 2023-2030.
The Infectious Disease Diagnostics Market refers to the market for diagnostic methods used to detect Infectious diseases, disorders caused by organisms such as bacteria, viruses, fungi, or parasites. The market includes tools and methods for detecting, analysing and interpreting the findings of diagnostic tests, imaging methods, and biomarkers.
The increased prevalence of Infectious diseases is significantly contributing to the growth of the global market for Infectious Disease Diagnostics Market. Advancements in diagnostic technologies are leading to more accurate and timely diagnosis of infectious diseases. This is driving the growth of the market.
Pharmaceutical and biotechnology firms like Thermo-Fisher Scientific Inc., Hologic, Inc., Abbott, Bio-Rad Laboratories, Inc., OraSure Technologies, Inc, F. Hoffmann-La Roche Ltd., Siemens Healthineers, Chembio Diagnostics, Inc., Danaher Corporation, and Becton, Dickinson and Company, are some of the major players in the Infectious Disease Diagnostics Market.
Infectious diseases are diagnosed using a variety of diagnostic procedures and tests, including assessments and imaging examinations like X-rays, CT scans, MRI scans, PET scans, and laboratory tests.
The growing prevalence of Infectious diseases and government measures to support its therapeutics are projected to drive the diagnostics market in the years to come. The market may yet have difficulties with regard to the higher cost of diagnostic tests and unfavourable reimbursement scenarios.
Market Dynamics
Drivers of France Infectious Disease Diagnostics Market:
Growing Incidence and Prevalence of Infectious Disease: As the prevalence of infectious diseases increases, the need for diagnostics will also rapidly increase which will boost the growth of the Infectious Disease Diagnostics Market significantly.
Increasing Demand for Point-of-Care-Testing (POCT): The focus of the population is shifting from laboratories to point-of-care-testing as it allows faster diagnosis. This will have a positive impact on the Infectious Disease Diagnostics Market.
Government measures and support: Initiatives by the government in providing facilities for advanced and better diagnostics services are attracting a lot of consumers and influencing the growth of the Infectious Disease Diagnostics Market.
Growing Interest in Personalised Medicine: The growing interest of people in individualised medicine will continue to fuel the development of the diagnostics market that can pinpoint specific biomarkers and genetic variants linked to Infectious diseases.
Restraints of France Infectious Disease Diagnostics Market:
Unfavourable Reimbursement Scenario: Insufficient reimbursement is a major reason hampering the growth of the Infectious Diseases Diagnostics market. A lot of companies face significant challenges in commercializing their tests; getting health insurers to pay for them.
High Price of Molecular-based Test Kits: The accessibility and adoption of molecular-based test kits is limiting due to the high costs of these tests.
Notable Deals in Infectious Disease Diagnostics Market:
In 2022, F. Hoffmann-La Roche Ltd launched a new dual antigen and antibody diagnostic test for hepatitis C which allows the simultaneous and independent determination of hepatitis C virus strain from a single human plasma or serum sample.
In 2022, SEEGENE received the CE-IVD mark from the EU regulatory authority for their Allplex SARS-CoV-2 rapid MDx Assay. This assay is appropriate for use in airports, schools, and other large-scale institutions since it has the capacity to produce results in less than 30 minutes.
Key players
bioM?rieux SA Roche Diagnostics Abbott Laboratories Siemens Healthineers BD (Becton, Dickinson and Company) Quidel Corporation Hologic, Inc. Thermo Fisher Scientific Inc. bioM?rieux Diagnostics Products Cepheid Inc. (a Danaher Corporation subsidiary)1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For France Infectious Disease Diagnostics Market
By Product:
- Assays
- Kits and Reagents
- Instruments
- Services and Software
By Test Type:
- Laboratory
- POC
By Disease:
- Covid-19
- HIV
- HAIs
- Hepatitis
- CT/ NG
- HPV
- TB
- Influenza
- Others
By Technology:
- Immunodiagnostics
- Clinical Microbiology
- PCR
- INAAT
- DNA Sequencing & NGS
- DNA Microarrays
- Others
By End-user:
- Hospitals & Clinics
- Diagnostic Laboratpries
- Academic Research Institutes
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.






































