France HIV Therapeutics Market Analysis
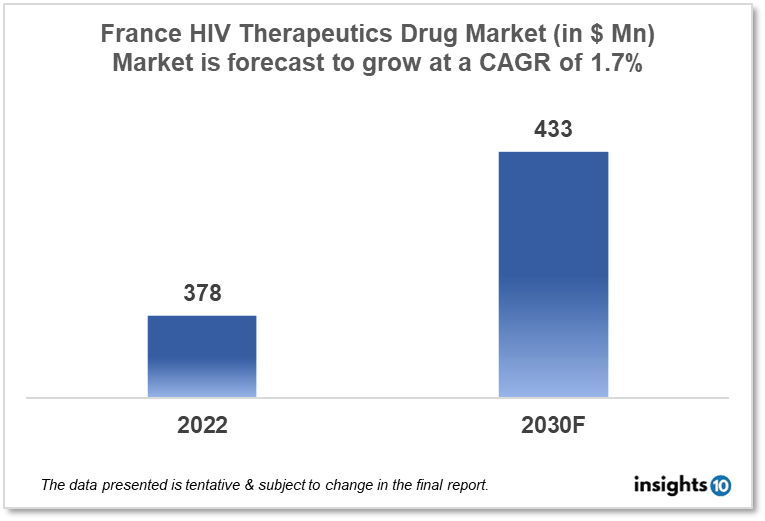
By 2030, it is anticipated that the France HIV therapeutics market will reach a value of $433.03 Mn from $378.40 Mn in 2022, growing at a CAGR of 1.7% during 2022-2030. The market is primarily dominated by local players such as Sanofi, Gilead Sciences France, and ViiV Healthcare France. The market is driven by various financial initiatives, increasing awareness campaigns, and the prevalence of the generic drug market. The HIV therapeutics market in France is segmented by type, product, geography, end user, and distribution channel.
Buy Now

France HIV Therapeutics Market Analysis Summary
By 2030, it is anticipated that the France HIV therapeutics market will reach a value of $433.03 Mn from $378.40 Mn in 2022, growing at a CAGR of 1.7% during 2022-2030.
The third-largest donor to the global AIDS response and a long-time UNAIDS partner, France is a champion of human rights, gender equality, important populations, education, and sexual and reproductive health and rights. France is the second-largest donor to the Global Fund to Fight AIDS, Tuberculosis, and Malaria and a founding member of the Global HIV Prevention Coalition. The Global Fund has received more than $6 Bn from France. Recently, a new strategic framework for cooperation and collaboration was signed by UNAIDS and the Global Fund to strengthen and expedite support for national efforts to end AIDS.
In 2019, France's healthcare system accounted for 11.1% of its GDP. The adult prevalence rate of HIV/AIDS in France as of the 2021 estimation is 0.3%. Comparatively speaking, France comes in at position 75. By offering free antiretroviral therapy (ART) to those in need via the public healthcare system, the French government has contributed significantly to the fight against the HIV/AIDS epidemic in the nation. The French government additionally finances initiatives related to HIV/AIDS in the private sector.
Market Dynamics
Market Growth Drivers Analysis
As a result of coordinated international efforts, including work done by civil society in France, 23.3 Mn of the world's 37.9 Mn HIV-positive people now has access to antiretroviral therapy. Early Access Programs are in place in France to permit the sale of specific pharmaceuticals that treat serious or uncommon diseases prior to discussions about reimbursement or pricing, especially for novel HIV therapies.
Market Restraints
The French HIV therapeutic market is fiercely competitive, and there are many generic medications that are offered at lower costs than name-brand medications. The market growth for name-brand drugs may be constrained by the fierce competition from generic medications. It can be challenging for businesses to quickly bring new drugs to market because the regulatory process for the approval of new HIV/AIDS drugs and therapies can be drawn out and complicated.
Competitive Landscape
Key Players
- Sanofi (FRA)
- Gilead Sciences France (FRA)
- ViiV Healthcare France (FRA)
- Abivax (FRA)
- Innate Pharma (FRA)
- Transgene (FRA)
- Moderna
- GlaxoSmithKline
Recent Notable Updates
January 2023: After the HIV vaccine was found to be ineffective at preventing infections, Johnson & Johnson announced that it was ending a late-stage global trial of the drug. This trial's failure comes more than a year after another of J&J's HIV vaccines failed a study, and it represents yet another setback in the quest for a vaccine against a virus known to mutate quickly and find novel ways to evade the immune system.
Healthcare Policies and Regulatory Landscape
One of France's competent authorities, the National Agency for the Safety of Medicines and Health Products (ANSM), is in charge of regulating the therapeutics market in that country. In France, the ANSM, an impartial public agency, is in charge of ensuring the efficacy, safety, and quality of medicines and medical devices. In order to guarantee that patients have access to safe and effective treatments, the ANSM also keeps an eye on the performance of the market and the pharmaceutical sector.
Reimbursement Scenarios
Early Access Programs are in place in France to permit the sale of specific pharmaceuticals that treat serious or uncommon diseases prior to discussions about reimbursement or pricing, especially for novel HIV therapies. In France, health insurance contributions are required for all citizens, and private insurance is optional for those who want more coverage. More than 75% of France's healthcare costs are covered by government-funded organizations.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
HIV Therapeutics Segmentation
By Types (Revenue, USD Billion):
- Nucleoside-Analog Reverse Transcriptase Inhibitors (NRTIs)
- Coreceptor Antagonists
- Entry and Fusion Inhibitors
- Integrase Inhibitors
- Protease Inhibitors (PIs)
- Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
By Distribution Channel (Revenue, USD Billion):
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































