France Hepatitis A Therapeutics Market Analysis
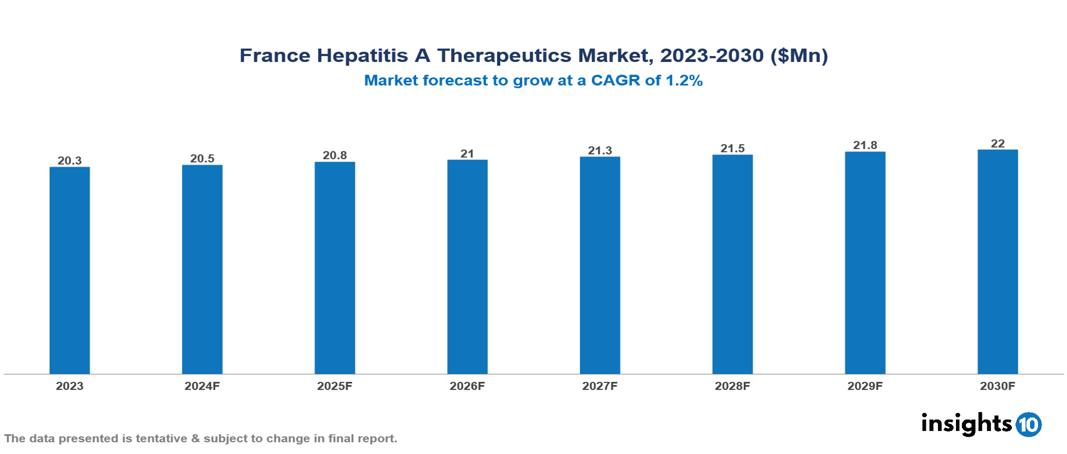
The France Hepatitis A Therapeutics Market was valued at $20.27 Mn in 2023 and is predicted to grow at a CAGR of 1.2% from 2023 to 2030 to $22.04 Mn by 2030. The market is propelled by increasing prevalence, globalization, travel trends, and government initiatives. Pharmaceutical companies like Merck & Co. Inc. and Zydus Cadila play pivotal roles in vaccine development and distribution, supporting France's public health endeavors.
Buy Now

France Hepatitis A Therapeutics Market Executive Summary
The France Hepatitis A Therapeutics Market was valued at $20.27 Mn in 2023 and is predicted to grow at a CAGR of 1.2% from 2023 to 2030 to $22.04 Mn by 2030.
Hepatitis A is a viral infection characterized by acute liver inflammation, with symptoms that can endure for several months yet typically resolve spontaneously without specific treatment. Transmission primarily occurs via the fecal-to-oral route. Hepatitis A induces liver inflammation with symptoms varying from mild to severe illness, categorized as an acute infection lasting up to two months. Compared to hepatitis B and hepatitis C, Hepatitis A is notably more contagious, often exhibiting noticeable symptoms during the infection period. However, it typically resolves without medical intervention. The prevalence of Hepatitis A is influenced by factors such as inadequate sanitation and contamination of food and water sources. The virus's high contagiousness enables it to persist in the environment without a host, facilitating its transmission through person-to-person contact and contributing to significant outbreaks within specific communities.
France has a growing infection rate of Hepatitis A. Factors increasing this growth include rising prevalence rates of Hepatitis A in France (27 cases per 100,000) and significant travel-associated cases (93 in 2021). The market is propelled by increasing prevalence, globalization, travel trends, and government initiatives, whereas limited treatment options, regulatory hurdles, and vaccine hesitancy restrain the market.
Market Dynamics
Market Growth Drivers
Increasing Prevalence: The higher incidence rates, persisting prevalence in blood donors, travel-associated cases, identified risk factors, and outbreaks have led to an increasing prevalence of Hepatitis A in France (27 cases per 100,000), significantly higher than in the US. In 2021, France reported 93 travel-associated Hepatitis A cases, accounting for two-thirds of all travel-related cases in the EU/EEA countries that year. This trend serves as a key market driver for Hepatitis A therapeutics in the country, emphasizing the need for effective treatments and preventive measures to address the growing burden of the disease.
Increased Globalization and Travel: Travel-related Hepatitis A has been responsible for around 46% of all cases in France, emphasizing the role of globalization and travels in transmitting the disease, thus driving demand for effective therapeutics.
Supportive Government initiatives: In France, cost-effectiveness analysis of prevention strategies, control measures, outbreak response, and surveillance programs are key drivers for Hepatitis A therapeutics. Agencies like Santé publique France emphasize vaccination to manage the disease burden. Surveillance programs for Hepatitis A guide vaccination policies, increasing the demand for therapeutics. These efforts foster a supportive environment for developing and adopting treatments to combat Hepatitis A in France.
Market Restraints
Limited Treatment Options: There are limited FDA-approved medications for Hepatitis A treatment. This restricts market growth, and specific data on the number of approved medications might be difficult to isolate for France.
Regulatory Hurdles: Regulatory hurdles, such as the need for comparative effectiveness and cost-effectiveness data, post-marketing commitments, and national-specific regulations, pose significant challenges for the Hepatitis A therapeutics market in France. Uncertainty surrounding the decision process and delays in issuing application decrees exacerbate these challenges. These regulatory barriers lead to delays and increased costs for drug approvals, restraining the growth of the Hepatitis A therapeutics market.
Vaccine Hesitancy: Vaccine hesitancy in France, fueled by historical controversies and political distrust, poses a significant market restraint for Hepatitis A therapeutics. The COVID-19 pandemic has worsened hesitancy with conflicting government messages. This skepticism impedes the adoption of Hepatitis A therapeutics in the market. High vaccine hesitancy in France, with around 25% unwilling to get vaccinated, poses a significant market restraint for the Hepatitis A therapeutics market, as communication strategies have failed to address this deep-rooted issue, impacting vaccine acceptance and uptake, thus restraining the market.
Regulatory Landscape and Reimbursement Scenario
The reimbursement scenario for Hepatitis A therapeutics in France is generally favourable, with a high likelihood of coverage prioritizing patient access to necessary treatments. The National Authority for Health (HAS) oversees reimbursement and pricing, assessing drugs based on medical evidence and therapeutic progress compared to existing therapies. Coverage is more likely for high-risk individuals, such as unvaccinated or incompletely vaccinated individuals with clinically significant infections and immunocompromised individuals.
Regulatory bodies like the National Agency for the Safety of Medicine and Health Products (ANSM) and the European Medicines Agency (EMA) ensure the quality, safety, and efficacy of medicines in France. Physicians may need to justify medication choices, and patients typically do not need to manage reimbursement directly, with most costs covered by the healthcare system. A small co-payment might be required, and healthcare providers are advised to prescribe treatments based on best practices and patient needs, staying informed about reimbursement policies and formularies.
Competitive Landscape
Key players
Here are some of the major key players in the Hepatitis A Therapeutics Market:
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- Zydus Cadilla
- Sanofi
- GlaxoSmithKline (GSK)
- Takeda
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Hepatitis A Therapeutics Market Segmentation
By Distribution Channel
- Hospital-based pharmacies
- Retail pharmacies
- Online pharmacies
By Route of Administration
- Oral Medications
- Intravenous Therapy
By Healthcare Setting
- Outpatient Care
- Inpatient Care
By Age
- Children
- Adults
- Senior Citizens
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.







































