France GMP Testing Service Market Analysis
France GMP Testing Service Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 - 2030. The market for GMP Testing Service is expanding as a result of rising demand of medical devices and increasing incidence of chronic illnesses. This demand is fueling the development of GMP Testing service market. Some of the key players in the global GMP Testing Service Market include Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Covance Inc., Nelson Laboratories LLC, Almac Group, Pace Analytical, Wuxi AppTec., Intertek Group PLC, and Charles River Laboratories.
Buy Now

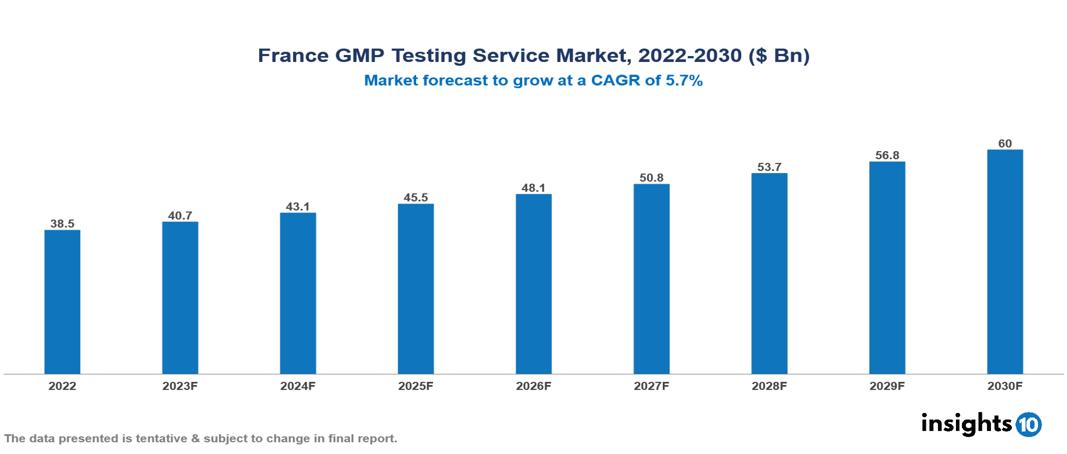
France GMP Testing Service Market Executive Summary
France GMP Testing Service Market is valued at around $38.5 Bn in 2022 and is projected to reach $60 Bn by 2030, exhibiting a CAGR of 5.7% during the forecast period 2023-2030.
In order to maintain a level of quality, safety, purity, and strength in any products that are distributed to the general public, GMPs are very crucial in the production of medical devices. Following these protocols helps producers prevent unfavourable outcomes, product recalls, and flaws that could later lead to expensive liability action. The importance of GMPs is rising as a result of the realisation that quality control and consistent testing conditions are essential for a productive production cycle and can help reduce product contamination, failure, and quality variation.
The emergence of biosimilars, combination products, and other novel medications has fueled the market for GMP Testing Service expansion by raising the demand for particular testing methods. Additionally, a number of biopharmaceutical firms use bioanalytical testing outsourcing services for assay validation and drug development at both the preclinical and clinical stages. As more patents expire, generic producers must conduct and submit the results of bioanalytical testing. This further fuels the market's expansion.
Pharmaceutical and biotechnology firms like Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Covance Inc., Nelson Laboratories LLC, Almac Group, Pace Analytical, Wuxi AppTec., Intertek Group PLC, and Charles River Laboratories, are some of the major players in the GMP Testing Service Market.
Due to the growing need for drug and device manufacturers to adhere to good manufacturing practices in their ongoing research and development activities, for the smooth approval of the target products by regulatory bodies, the market for GMP Testing Services is predicted to experience healthy growth in the upcoming years. Future demand for GMP testing services is anticipated to increase as a result of major pharmaceutical and biopharmaceutical firms' increased emphasis on the development of multiple therapies and medical devices intended to offer effective treatment for a range of chronic conditions.
The advancements in technologies such as the advent of chromatography, spectroscopy, and spectrometry are projected to fuel the market for GMP Testing Services in the years to come. The market may yet face difficulty in expansion due to the precise regulatory framework requirements for GMP outsourced activities.
Market Dynamics
Drivers of France GMP Testing Service Market:
Rising Demand for Medical Devices: Growing demand for gadgets that are linked to the patient's EMR, wearables, telephones, and telemedicine platforms to deliver essential data for better clinical and operational decisions are driving the growth of the market for GMP Testing Services.
Increasing Chronic Illnesses: The two main factors anticipated to affect the medical device industry's advancement and, hence, influence the growth of the market for GMP Testing Services are the increase in the prevalence of chronic illnesses and the rising older population.
Technological Advancements: The integration of medical equipment with the Internet of Medical Things (IoMT) systems uses smart devices, smart sensors, and other portable communication tools. These developments aid medical practitioners in better patient outcomes while lowering expenses and increasing productivity, boosting the growth of the market for GMP Testing Services.
Developments in GMP Testing Service Market:
In 2022, Almac Diagnostic Services, a subsidiary of the Almac Group, inked a Master Collaboration Agreement (MCA) with AstraZeneca to develop and market a number of companion diagnostic (CDx) products. Almac will work with AstraZeneca to provide novel treatments to patients in places with significant unmet medical needs.
In 2022, Almac Group announced an investment worth $ 536682.50 in NMR technology to enhance the security, flexibility, and capacity of its analytical services. High-resolution NMR is a key technology that delivers advanced characterization and purity assessment abilities, meeting all necessary regulatory requirements. The service helps deliver comprehensive solutions to support active pharmaceutical ingredient (API) and drug product development programs, from the early phases to commercialization.
In 2022, Eurofins Scientific acquired a majority stake in the QSAI Analysis and Research Center Co., Ltd. (QARC). The acquisition will complement the Eurofins network's extensive food testing capabilities with pesticides, veterinary drugs, foreign objects, and sensory testing services. The acquisition will further strengthen Eurofins' presence in Japan and the Eurofins network's offering to customers importing and exporting food products.
Key players
Eurofins Scientific Bureau Veritas SGS SA Charles River Laboratories International, Inc. Intertek Group plc LGC Limited ALS Limited Tentamus Group Laboratoire Dubois Bioalternatives1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For France GMP Testing Service Market
By Service Type:
- Product validation Testing
- Bionalytical Services
- Packaging and Shelf-life Testing
- Others
By End-user:
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Medical Devices Company
- Cosmetic Industry
- Food & Beverages Industry
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.










































