France Glioblastoma Multiforme Therapeutics Market Analysis
France Glioblastoma Multiforme Therapeutics Market is projected to grow from $xx Mn in 2023 to $xx Mn by 2030, registering a CAGR of xx% during the forecast period of 2023 ? 2030. The most aggressive primary brain tumour is glioblastoma, also known as glioblastoma multiforme (GBM), which makes up for 15% of all intracranial neoplasms and 49% of malignant brain tumours. GBM is more common and has a worse prognosis as people age. It is still very difficult to increase the overall survival (OS) and progression-free survival (PFS) of GBM patients. Consequently, the GBM market has drivers. It is still very difficult to increase the overall survival (OS) and progression-free survival (PFS) of GBM patients. Global The following companies are significant players in the market: Arbor Pharmaceuticals, LLC, Amneal Pharmaceuticals, Karyopharm Therapeutics, Inc., Sumitomo Dainippon Pharma Oncology, Inc., Merck & Co., Inc., Amgen, Inc., F. Hoffmann-La Roche Ltd., Pfizer Inc., Amgen, Inc., Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd. (Boston Biomedical, Inc.)
Buy Now

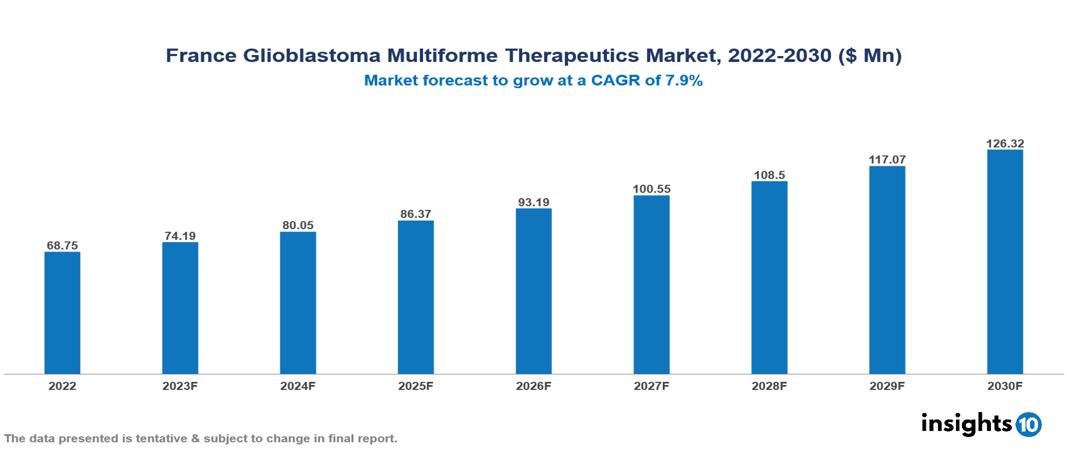
France Glioblastoma Multiforme Therapeutics Market Analysis Summary
France Glioblastoma Multiforme Therapeutics Market is valued at around $68.75 Mn in 2022 and is projected to reach $126.32 Mn by 2030, exhibiting a CAGR of 7.9% during the forecast period 2023-2030.
The most aggressive glial tumour of the central nervous system is called glioblastoma multiforme (GBM). GBM is an aggressive subtype of gliomas of the central nervous system with a poor prognosis. Although GBM accounts for more than 60% of all adult brain tumours and is the most common kind of malignant glioma, its overall incidence is rare, occurring at a rate of 3.21 per 100,000 people. Although it can happen at any age, the peak incidence occurs in those between the ages of 55 and 60. Men are more likely than women to get GBM. Although little is known regarding the aetiology of GBM, one theory put out suggests that the disease's pathogenesis may be connected to a chronic inflammatory process that was set off by traumatic brain damage.
GBM is more common and has a worse prognosis as people age. It is still very difficult to increase the overall survival (OS) and progression-free survival (PFS) of GBM patients. Consequently, the GBM market has drivers. It is still very difficult to increase the overall survival (OS) and progression-free survival (PFS) of GBM patients.
Global Merck & Co., Inc., Amgen, Inc., F. Hoffmann-La Roche Ltd., Pfizer Inc., Amgen, Inc., Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Ltd., Arbor Pharmaceuticals, LLC, Amneal Pharmaceuticals, Karyopharm Therapeutics, Inc., and Sumitomo Dainippon Pharma Oncology are important players in the market.
Market Dynamics
Market Drivers
With advancing age, GBM is more common and has a worse prognosis. For individuals with GBM, it is still very difficult to increase both overall survival (OS) and progression-free survival (PFS). Therefore, the market for GBM is driven.
Market Development
The drug Paxalisib is being developed by Kazia Therapeutics for the treatment of solid tumours, juvenile diffuse intrinsic pontine glioma, high-grade glioblastoma or glioma, breast cancer brain metastases, and many other conditions.
New Phase 1B Clinical Trial for Glioblastoma Multiforme is Started by Chimeric Therapeutics.
Alaunos Therapeutics is currently in Phase II of its TCR-T Library clinical trial for glioblastoma multiforme (GBM).
The U.S. FDA granted fast-track designation to Denovo Biopharma's DB102 (enzastaurin) in July 2020 for the treatment of people who have just received a glioblastoma diagnosis.
Market Restraints
It is still very difficult to increase the overall survival (OS) and progression-free survival (PFS) of GBM patients. Consequently, the GBM market has drivers. Patients who get the standard first-line therapy, which entails surgery, concurrent radiochemotherapy, and maintenance chemotherapy, bear a high financial burden. Glioblastoma multiforme (GBM) cells embed themselves in brain tissue, which makes it challenging to eradicate them with standard treatments like surgery, radiation, and chemotherapy. This is one of the challenges in treating brain cancer. Additionally, because they are "cold" tumours, immunotherapies cannot effectively target them because they lack immune cells.
Key players
Roche Novartis Genentech Bristol-Myers Squibb Merck AbbVie Sanofi Pfizer Celgene Johnson & Johnson
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Market Segmentations For France Glioblastoma Multiforme Therapeutics Market
By Treatment
-
By Therapy
-
Radiation therapy
-
Chemotherapy
-
Surgery
-
Drugs
-
Bevacizumab
-
Temozolomide
-
Everolimus
-
Corticosteroids
-
5- aminolevulinic acid
By End Users
-
Hospitals
-
Clinics
-
Surgical centres
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































