France Digital Therapeutics Market Analysis
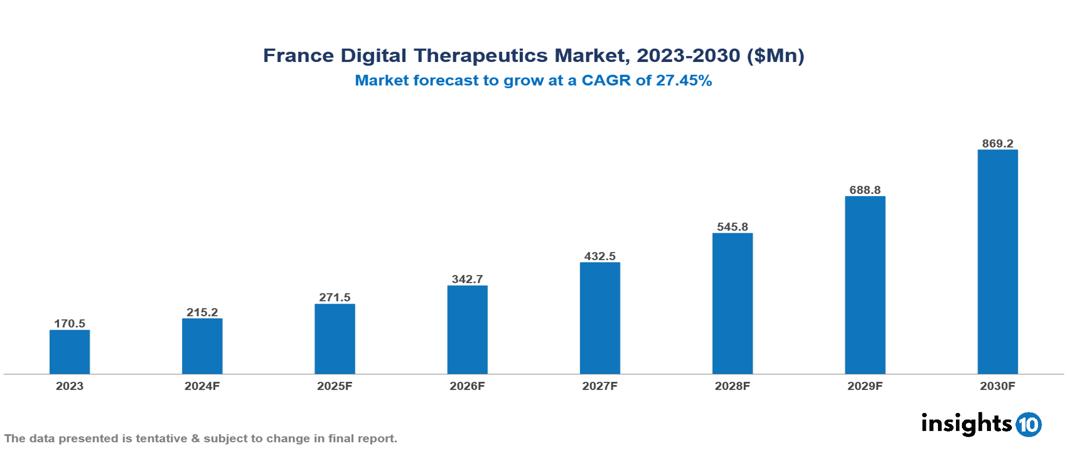
France Digital Therapeutics Market is at around $0.17 Bn in 2023 and is projected to reach $0.9 Bn in 2030, exhibiting a CAGR of 26.2% during the forecast period. The market's expansion is being driven by government initiatives and policies, technological advancements, and an aging population. The market is dominated by key players like Mindmaze, Theravance, Voluntis, Omada Health Inc., 2Morrow Inc., Livongo Health Inc., WellDoc, Propeller Health, Canary Health, Noom Inc., Akili Interactive Labs Inc., and HYGIEIA.
Buy Now

France Digital Therapeutics Market Executive Summary
France Digital Therapeutics Market is at around $0.17 Bn in 2023 and is projected to reach $0.9 Bn in 2030, exhibiting a CAGR of 26.2% during the forecast period.
The French market for digital therapeutics is a quickly expanding area of the healthcare industry that uses digital technologies to provide evidence-based treatment solutions. This market includes a wide range of digital solutions for managing and treating different medical diseases, such as wearable technology, software, and mobile apps. The French Digital Therapeutics Market illustrates the nation's dedication to promoting cutting-edge healthcare solutions in the digital era, with an emphasis on utilizing technology to improve patient outcomes and lower healthcare costs.
The market for digital therapeutics in France is expanding rapidly, mostly due to the growing utilization of digital health solutions. The growing incidence of chronic illnesses and government programs encouraging healthcare digitization fuel market growth. Major players are actively forming alliances and working together to expand their market share and satisfy the rising need for cutting-edge digital therapy solutions.
The global market for digital therapeutics has shown phenomenal growth, with revenue of $6.2 Bn in 2023. The notable growth observed in this sector is indicative of the profound transformation brought about by the application of advanced technology and cost-effective production methods. Digital treatments are becoming more and more important in the healthcare system as a result. The market's current trend suggests that more changes could occur, and it is anticipated that continuing innovations will support the industry's continued expansion.
MindMaze is a prominent participant in the worldwide digital therapies industry, with a particular emphasis on France. To provide its digital neurotherapeutics solutions to patients in France, MindMaze has worked with prominent French institutions like as Swiss Rehabilitation and Guttmann Barcelona. These collaborations give access to clinical knowledge and distribution networks. The emphasis MindMaze places on neurorehabilitation and neurorestoration is in line with the aging population of France and the growing need for cutting-edge therapies for neurological diseases such as multiple sclerosis, Parkinson's disease, and stroke. The company now has the means for additional growth and French commercialization owing to a recent $125 Mn fundraising round.
Market Dynamics
Market Growth Drivers:
Technological Developments: The creation of increasingly complex and individualized digital therapies is facilitated by continuous technological breakthroughs, particularly in fields like wearable technology, machine learning, and artificial intelligence.
Government programs and Policies: The market is expanding with supportive government policies and programs that encourage the adoption of digital health solutions, such as digital therapies.
Growing Aging Population: As people age, they become more vulnerable to long-term illnesses, which expands the target market for digital therapies. Elderly people are especially in need of remote monitoring and management of their health issues.
Market Restraints:
Regulatory obstacles include strict procedures and a lack of assurance regarding health authorities' approval of digital therapies, which can stifle industry expansion.
Reimbursement Issues: Patients and healthcare providers may be discouraged from implementing digital treatments due to unclear or restricted reimbursement policies.
Concerns about Data Security and Privacy: Concerns over patient privacy and security may pose a serious barrier to the widespread use of digital therapies, given the sensitive nature of health data.
Healthcare Policies and Regulatory Landscape
Agence nationale de sécurité du médicament et des produits de santé (ANSM) is run by the Ministry of Health. On behalf of the French government, it guarantees the security of medical supplies and gives access to state-of-the-art therapies. ANSM inspectors are authorized to conduct audits and inspections to ensure compliance with relevant laws and regulations, as well as to apply various enforcement actions. A more complex centralized approvals process that necessitates compliance with both national and EU regulatory criteria is made possible by the European Medicines Agency (EMA) cooperation. The negotiation of payment after approval adds to the overall intricacy of the French approval procedure.
Competitive Landscape
Key Players:
- Mindmaze
- Theravance
- Voluntis
- Omada Health Inc.
- 2Morrow Inc.
- Livongo Health Inc.
- WellDoc
- Propeller Health
- Canary Health
- Noom Inc.
- Akili Interactive Labs Inc.
- HYGIEIA
1. Executive Summary
1.1 Digital Health Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Digital Health Policy in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Digital Therapeutics Market Segmentation
By Solution
- Software
- Services
By Deployment
- Cloud-based
- On-premises
By Application
- Therapy
- Diabetes
- Obesity
- CNS
- Respiratory
- CVD
By End-use
- Diagnostic Centres
- Healthcare Players
- Healthcare Research Centres
- Hospitals & Clinics
- Nursing Care Centres
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































