France Contraceptive Devices Market Analysis
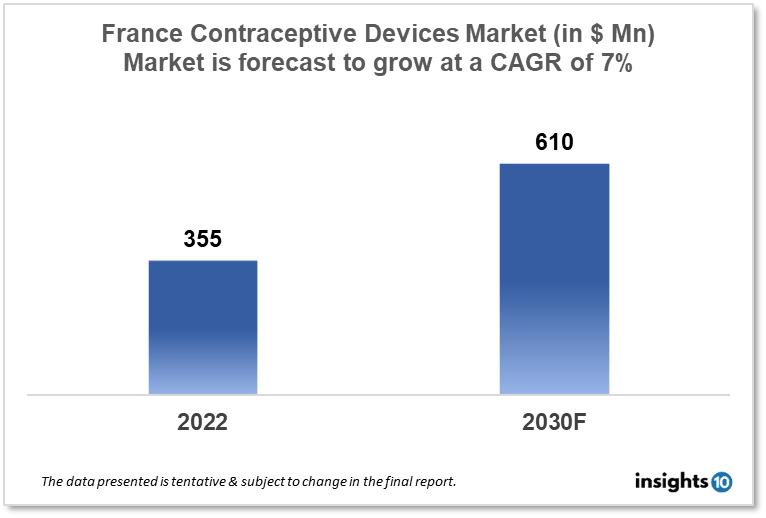
France's Contraceptive Devices Market is expected to witness growth from $355 Mn in 2022 to $610 Mn in 2030 with a CAGR of 7.00% for the forecasted year 2022-30. To encourage family planning and contraception, the French government has put regulations into place, such as offering free contraception to women under the age of 25. This support is boosting the demand for contraceptive methods and opening up businesses for manufacturers. The market is segmented by type and by gender. Some key players in this market include Laboratoire CCD, HRA Pharma, Mylan Laboratories, Church & Dwight, CooperSurgical, Pfizer, Pregna International, and Teva Pharmaceutical.
Buy Now

France Contraceptive Devices Healthcare Market Executive Analysis
France's Contraceptive Devices Market is expected to witness growth from $355 Mn in 2022 to $610 Mn in 2030 with a CAGR of 7.00% for the forecasted year 2022-30. Healthcare expenditures in France total $5,370 per person or 12.3% of the country's GDP. It is anticipated that France's population will rise in 2023 by 0.36%. In Germany, men can anticipate a 79.53-year life expectancy, compared to a woman's 85.79-year life expectancy. France spent $5,370 on health care in 2022, a decrease of 3.56% from 2020 to 2022. 9.26% of all health care spending is made up of personal costs.
In France, there were 267,097 cases of sexually transmitted infections that were reported in 2021. Chlamydia, gonorrhea, and syphilis were the most prevalent sexually transmitted infections, particularly among young individuals, the prevalence of STIs has been rising recently. 87 maternal deaths occurred in France between 2019 and 2022, translating to a maternal mortality ratio of 7.1 deaths per 100,000 live births. One of the best ways to prevent unwanted pregnancy is with contraceptive devices. France offers a wide range of contraceptive methods, including condoms, hormonal contraceptives (including the pill, patch, and ring), intrauterine devices, and implants. Choosing when or if to have children gives people more control over their reproductive health thanks to contraceptive methods. People who desire to postpone getting pregnant, space out their pregnancies, or avoid getting pregnant entirely may find this to be very crucial. Hormonal contraceptives are one example of a contraceptive method that can provide health advantages in addition to preventing conception. Hormonal contraceptives, for instance, can help control menstrual cycles, lessen menstrual discomfort and bleeding, and increase cancer prevention. Some contraceptive methods, including condoms, can also aid in reducing the risk of sexually transmitted diseases being spread during sexual activity.
Market Dynamics
Market Growth Drivers
In order to encourage family planning and contraception, the French government has put regulations into place, such as offering free contraception to women under the age of 25. This support is boosting the demand for contraceptive methods and opening up businesses for manufacturers. Significant technological improvements are being made in the French market for contraceptive devices, including the creation of long-acting reversible contraceptives and the application of digital health technology to enhance contraceptive use. These developments are increasing the demand for cutting-edge and novel contraceptive methods. In France, women are becoming more independent and taking charge of their reproductive health. As a result, there is a rising desire for contraceptive methods that provide users with more freedom and control over their reproductive decisions.
Market Restraints
Despite increased understanding and acceptance of the practice, France's general population still occasionally stigmatises utilising contraception. For some people, adoption may be challenging, especially in conservative or religious countries. Contraceptive devices, like any medical equipment, may have unfavourable side effects or design flaws that necessitate product recalls. These mistakes could harm the reputation of the company and erode consumer confidence in the product. Manufacturers fiercely compete for market share in the French market for contraceptive devices. Price reductions as a result of this competition can affect producer profitability.
Competitive Landscape
Key Players
- Laboratoire CCD (FR)
- HRA Pharma (FR)
- Mylan Laboratories
- Church & Dwight
- CooperSurgical
- Pfizer
- Pregna International
- Teva Pharmaceutical
Healthcare Policies and Regulatory Landscape
In France, The French regulatory body, the National Agency for the Safety of Medicines and Health Products (ANSM), oversees contraceptive devices. Before being permitted for sale in the French market, the ANSM makes sure that these devices meet safety and efficacy requirements. In France, a healthcare professional's prescription is necessary for the majority of contraceptive methods. This guarantees that the gadget is suitable for the person's medical requirements and that they receive the necessary counselling regarding its use. In France, the government reimburses the cost of several contraceptive methods through the public healthcare system. This covers tablets, implants, contraceptive patches, and intrauterine devices. Advertising for prescription drugs, including contraceptive methods, is legally prohibited in France. It is against the law to advertise prescription drugs to the general public. The French government has made sex education a top priority, and it is mandated that schools teach it to kids. The usage of contraceptive techniques and their advantages are covered in this instruction.
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Contraceptive Devices Market Segmentation
By Type (Revenue, USD Billion):
A condom is a sheath-shaped barrier device used during sexual intercourse to reduce the probability of pregnancy or a sexually transmitted infection (STI). There are both male and female condoms. With proper use, women whose partners use male condoms experience a 2% per-year pregnancy rate. Their use greatly decreases the risk of gonorrhea, chlamydia, trichomoniasis, hepatitis B, and HIV/AIDS, per the World Health Organization Updates in November 2020. To a lesser extent, they also protect against genital herpes, human papillomavirus (HPV), and syphilis. Hence, the other reasons that aid the growth of the contraception devices market are the rising occurrence of STDs and the substantial increase in the population
- Condoms
- Diaphragms
- Cervical Caps
- Sponges
- Vaginal Rings
- Intra Uterine Devices (IUD)
- Other Devices
By Gender (Revenue, USD Billion):
Based on Gender the contraceptive devices market is segmented into male and female.
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































