France Constipation Therapeutics Market Analysis
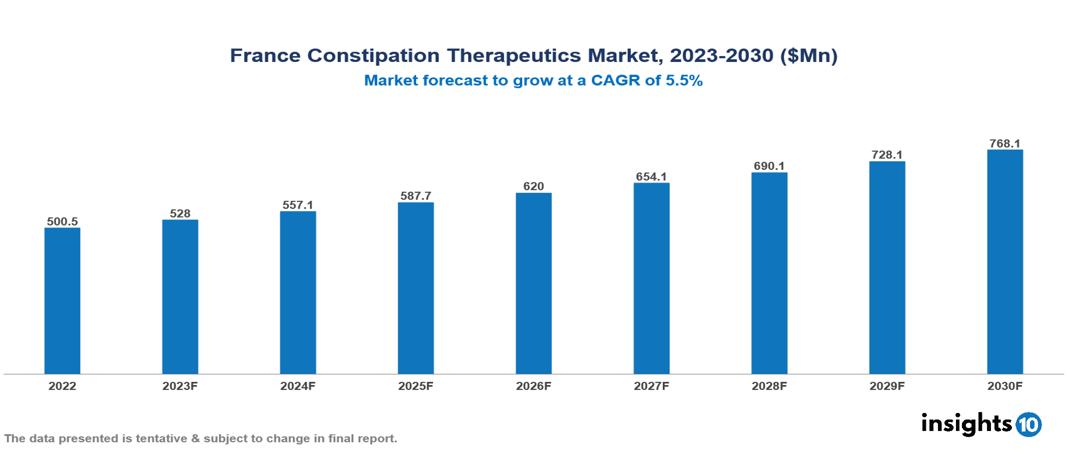
France Constipation Therapeutics Market was valued at $501 Mn in 2022 and is estimated to reach $768 Mn in 2030, exhibiting a CAGR of 5.5% during the forecast period. The constipation therapeutics market is experiencing growth driven by rising global rates of constipation associated with factors like aging, sedentary lifestyles, and poor dietary habits, as well as the expanding elderly population, which is particularly prone to chronic conditions. Notable participants in this industry include AbbVie, Almirall, Boehringer Ingelheim, Ferring Pharmaceuticals, Ipsen Pharma, Johnson & Johnson, Norgine Pharmaceuticals, Pierre Fabre Médicament, Recordati and Sanofi.
Buy Now

France Constipation Therapeutics Market Executive Summary
France Constipation Therapeutics Market was valued at $501 Mn in 2022 and is estimated to reach $768 Mn in 2030, exhibiting a CAGR of 5.5% during the forecast period.
Constipation is a digestive condition characterized by irregular bowel movements, difficulty in stool passage, or the discharge of hard and dry stool. This condition arises when the transit of stool through the colon (large intestine) slows down, resulting in increased water absorption and stool hardening. Typical symptoms of constipation include straining during bowel movements, a feeling of incomplete evacuation, abdominal discomfort, and irregular or infrequent bowel habits. Multiple factors, such as a low-fiber diet, insufficient fluid intake, lack of physical activity, specific medications, and underlying medical issues, can contribute to constipation. Addressing constipation often involves lifestyle modifications, dietary changes, increased physical activity, and, when necessary, the use of medications to manage and alleviate symptoms.
Constipation is a notable health concern in France, with a prevalence of 22.4% among individuals aged 15 years and older. Notably, factors such as female gender, age, and a history of vaginal delivery in women are significantly associated with an increased likelihood of experiencing constipation. The prevalence of functional constipation specifically stands at 14.5%. Contributing to these figures are various factors, including dietary habits and lifestyle choices. The renowned French diet, characterized by its emphasis on rich and varied foods, may sometimes lack sufficient fiber, thereby contributing to constipation. Additionally, sedentary lifestyles and a lack of physical activity further exacerbate the issue. Managing and addressing constipation in France involves a multifaceted approach, incorporating lifestyle modifications, dietary adjustments, and potential therapeutic interventions, with healthcare providers actively working to raise awareness about effective constipation management strategies.
Innovative methods are growing increasingly prevalent in the field of constipation treatments. Drugs that target the serotonin 4 (5-HT4) and guanylate cyclase C (GC-C) receptors are showing promise in clinical trials as novel ways to treat constipation. These novel pharmacological interventions offer promising directions for further treatment options. Concurrently, current investigations examine the complex function of the gut microbiome in constipation. The goal of studying microbiome-based therapies is to improve bowel function by altering the gut microbiota. This evolving area of study holds promise for developing tailored treatments that harness the potential of the microbiome to alleviate constipation, presenting exciting possibilities for the future of constipation management.
Market Dynamics
Market Growth Drivers
Aging population: The aging population in France, projected to reach 29% aged 65 and over by 2050, acts as a key growth driver for the constipation therapeutics market. This demographic, experiencing higher constipation rates due to reduced physical activity, dietary changes, and medication side effects, creates a rising demand for effective therapeutic interventions. The need for tailored solutions for the elderly population presents a significant market opportunity, fostering growth in constipation therapeutics as healthcare providers and pharmaceutical companies address the specific needs of this expanding demographic.
Changing Dietary Habits and Sedentary Lifestyles: The rising prevalence of constipation treatment in France can be attributed to shifts in dietary patterns and a more sedentary way of life. Elements like inadequate fast-food consumption, low fiber intake, and decreased physical activity are linked to constipation. Additionally, the COVID-19 pandemic and subsequent lockdown measures have resulted in the emergence of new constipation symptoms, underscoring the influence of lifestyle alterations on digestive well-being.
Improvements in Healthcare Infrastructure: Enhancements in the healthcare infrastructure have resulted in improved identification and management of constipation, thereby fostering market expansion. The availability of skilled healthcare professionals, a high incidence of irritable bowel syndrome (IBS) and chronic constipation, and advancements in pharmaceuticals and treatment techniques have brought about a transformation in the constipation treatment market.
Market Restraints
Sociocultural Factors: Sociocultural factors, including constipation stigma and reliance on traditional remedies, pose a significant restraint on the constipation therapeutics market. The stigma leads to hesitancy in seeking medical assistance, while the preference for traditional remedies can cause delays in proper diagnosis and treatment. Overcoming these barriers requires tailored educational initiatives and the development of therapeutics aligned with local practices to enhance their effectiveness in the market.
Regulatory Environment: The regulatory environment presents challenges for the constipation therapeutics market. The prolonged and costly process of obtaining approval for new medications delays their market availability while navigating intricate reimbursement procedures can impede access for both healthcare institutions and patients. This regulatory landscape acts as a significant restraint, affecting the timely introduction and accessibility of specific constipation medications in the market.
Cost and Affordability: Cost and affordability pose significant challenges for the constipation therapeutics market. The high expenses associated with newer medications limit accessibility, particularly for those lacking comprehensive insurance coverage. Unequal access to healthcare, especially in rural areas, exacerbates the issue, hindering timely diagnosis and treatment of constipation. Additionally, even with insurance, elevated out-of-pocket costs for specific medications or laxatives act as barriers for some patients, impacting market penetration and growth.
Healthcare Policies and Regulatory Landscape
In France, healthcare policies and the regulatory landscape for pharmaceuticals are managed by institutions such as the Haute Autorité de Santé (HAS) and the French National Agency for Medicines and Health Products Safety (ANSM). HAS evaluates the effectiveness and cost-effectiveness of healthcare interventions, while ANSM oversees the safety of medicines. The Pricing and Reimbursement Committee (CEPS) engages in negotiations to establish drug prices and ensures economic viability. The Transparency Commission, operating under HAS, assesses the therapeutic value of drugs and provides recommendations for reimbursement. The funding of healthcare, including drug reimbursement, is significantly influenced by the French Social Security System. In addition, adherence to European standards is imperative, necessitating approval from the European Medicines Agency (EMA) for marketing authorization. This comprehensive framework is designed to maintain rigorous standards for drug safety, efficacy, and affordability within the context of the French healthcare system.
Competitive Landscape
Key Players
- AbbVie
- Almirall
- Boehringer Ingelheim
- Ferring Pharmaceuticals
- Ipsen Pharma
- Johnson & Johnson
- Norgine Pharmaceuticals
- Pierre Fabre Médicament
- Recordati
- Sanofi
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Constipation Therapeutics Market Segmentation
By Therapeutic
- Laxatives
- Chloride Channel Activators
- Peripherally Acting Mu-Opioid Receptor Antagonists
- GC-C Agonists
- 5-HT4 Receptor Agonists
By Disease
- Chronic Idiopathic Constipation
- Irritable Bowel Syndrome with Constipation
- Opioid-Induced Constipation
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.