France Compression Therapy Market Analysis
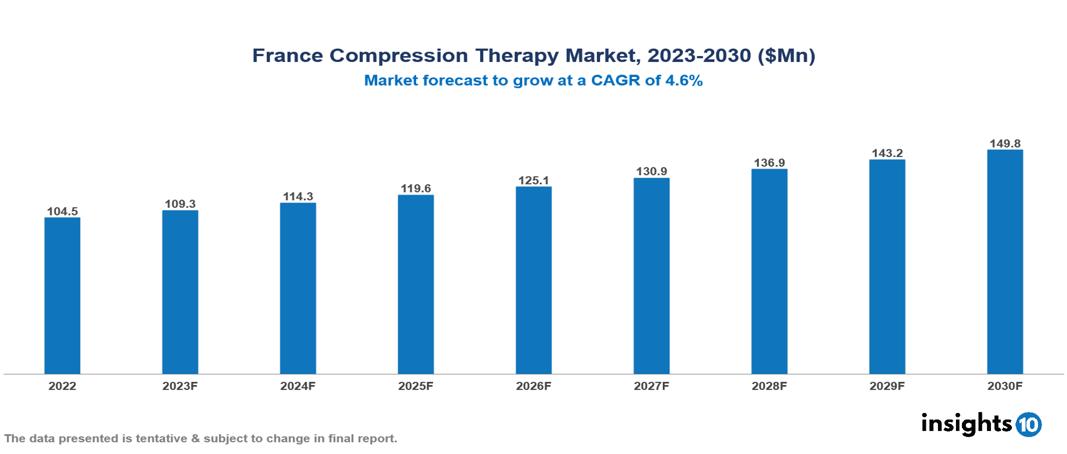
France Compression Therapy Market was valued at $105 Mn in 2022 and is estimated to reach $150 Mn in 2030, exhibiting a CAGR of 4.6% during the forecast period. The increase in the occurrence of venous conditions such as leg ulcers, deep vein thrombosis, lymphedema, varicose veins, and blood clots is a crucial factor contributing to the expansion of the compression therapy market. Major participants in this sector include companies like Medi, Sigvaris, Juzo, Jobst, Varisan, Physiance, Thuasne, DJO Global, FLA Orthopedics, and Bauerfeind.
Buy Now

France Compression Therapy Market Executive Summary
France's Compression Therapy Market was valued at $105 Mn in 2022 and is estimated to reach $150 Mn in 2030, exhibiting a CAGR of 4.6% during the forecast period.
Compression therapy is a medical procedure commonly utilized on limbs to enhance blood circulation and reduce swelling. It is often employed in the treatment of conditions like lymphedema, venous disorders, and certain types of edemas. The primary objective is to enhance the flow of blood in the veins, facilitating the return of blood to the heart. This helps prevent the accumulation of fluid in the tissues, especially when swelling is a result of fluid retention. Depending on the severity of the condition and the individual's specific needs, compression therapy can take various forms, such as elastic stockings, sleeves, or bandages. Techniques like compression wraps involve the use of multiple layers of bandages to provide graduated compression, while intermittent pneumatic compression (IPC) employs a device to mimic natural muscle contractions, assisting in venous return. To determine the most suitable technique and compression level for a particular case, it is essential to consult with a healthcare provider, especially when developing comprehensive treatment plans for issues like poor circulation, swelling, or fluid retention.
In France, chronic venous insufficiency (CVI) is a significant health issue that affects between 30% and 35% of adults. Gender disparity is evident in the gender-specific pattern of varicose veins, which affect 30.1% of men and a significantly higher 50.5% of women. The annual incidence of deep vein thrombosis (DVT) is one case per 1,000 people. The elevated rates of venous disorders can be attributed to some factors, including lifestyle-related ones like increased obesity prevalence, prolonged periods of sitting or standing, and decreased physical activity. An important contributing factor is also found to be genetic predisposition, with some populations having a higher risk of venous disorders. Pregnancy-related physiological changes and a family history of vein problems also raise a person's risk, particularly for women. These results highlight the complex nature of CVI and the significance of managing and preventing venous disorders in the population by taking genetic and lifestyle factors into account.
Companies like Sigvaris and Medi are pioneering compression therapy innovation by incorporating sensors into socks and stockings. This technology monitors vital signs for tailored treatment and enables remote patient monitoring. Advanced materials, such as microfibers and breathable textiles, enhance the effectiveness and comfort of compression garments, improving the overall user experience and therapy compliance.
Market Dynamics
Market Growth Drivers
Aging Population: The aging population in France, currently at 20.5% and projected to reach 29% by 2050 and 35% by 2070, is a key driver for the compression therapy market. The higher incidence of venous disorders and lymphedema among the elderly fuels the demand for compression therapy products, fostering market growth.
Prevalence of Chronic Diseases: The prevalence of chronic diseases in France, with 17% of the population being obese (BMI 30 or above), drives growth in the compression therapy market. This includes managing conditions like venous ulcers and deep vein thrombosis associated with diseases such as diabetes and obesity. As these cases increase, so does the demand for compression therapy, fostering market growth in France.
Awareness and Education: The increasing recognition of the advantages of compression therapy among both healthcare professionals and patients is playing a pivotal role in driving market expansion in the compression therapy market. As awareness grows, there is a heightened acceptance of compression therapy, leading to an increased demand for these products and contributing significantly to market growth.
Market Restraints
Cost Barriers: The elevated expenses linked to compression therapy products can pose a constraint on market growth. This is primarily due to the potential limitation of accessibility, especially among individuals with lower income levels, which hinders their ability to afford these products. Consequently, the high costs act as a deterrent, impeding the widespread adoption of compression therapy and limiting the market's expansion.
Inconvenience and Discomfort: The discomfort or inconvenience experienced by certain individuals when wearing compression garments can result in non-compliance and a decreased willingness to integrate compression therapy into their daily routine. This becomes a market restraint as it hinders widespread acceptance and usage of compression therapy, impacting the overall growth of the market.
Inconsistent Patient Adherence: The effectiveness of compression therapy is contingent upon consistent and correct usage. If patients deviate from the prescribed usage regimens, the therapy may lose its effectiveness, which can negatively impact the growth of the compression therapy market. This inconsistency in patient adherence acts as a market restraint by potentially undermining the therapeutic outcomes and reducing overall market demand.
Healthcare Policies and Regulatory Landscape
The French National Agency for Medicines and Health Products Safety (ANSM) and the Haute Autorité de Santé (HAS) regulate healthcare policies and regulations concerning therapeutic drugs in France. While ANSM is in charge of regulating and supervising the safety of medications, HAS evaluates the cost- and effectiveness-effectiveness of healthcare interventions. To maintain economic viability, the Pricing and Reimbursement Committee (CEPS) negotiates drug prices. A division of HAS, the Transparency Commission assesses medications' therapeutic value and makes recommendations regarding reimbursement. The French Social Security System is essential to the financing of healthcare, including the payment for prescription drugs. Marketing authorization requires approval from the European Medicines Agency (EMA) due to the importance of adhering to European standards. This all-encompassing framework is intended to uphold strict guidelines for medication efficacy, safety, and affordability in the context of the French healthcare system.
Competitive Landscape
Key Players
- Medi
- Sigvaris
- Juzo
- Jobst
- Varisan
- Physiance
- Thuasne
- DJO Global
- FLA Orthopedics
- Bauerfeind
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Compression Therapy Market Segmentation
By Product
- Compression Bandages
- Compression Wraps
- Compression Stockings
- Compression Tapes
- Compression Pumps
- Compression Braces
- Other Compression Garments
By Technique
- Static Compression Therapy
- Dynamic Compression Therapy
By Application
- Varicose Vein Treatment
- Deep Vein Thrombosis Treatment
- Lymphedema Treatment
- Leg Ulcer Treatment
- Other Applications
By Distribution Channel
- Pharmacies and Retailers
- Hospitals and Clinics
- E-Commerce Platforms
- Home Care Settings
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.