France Clinical Nutrition for Cancer Care Market Analysis
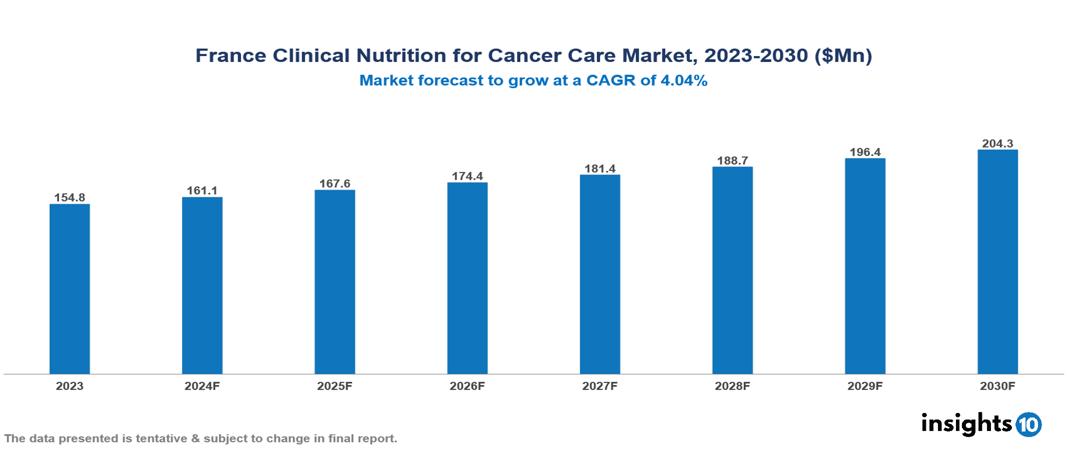
The France Clinical Nutrition for Cancer Care Market was valued at $154.8 Mn in 2023 and is predicted to grow at a CAGR of 4.04% from 2023 to 2030, to $204.3 Mn by 2030. The key drivers of the market include the increasing prevalence of cancer, increased awareness and education, and increasing healthcare expenditure. The prominent players of the France Clinical Nutrition for Cancer Care Market are Danone Nutricia, Laboratories Lactalis Nutrition Santé (LNS), Nestlé Health Science, Abbott Nutrition, B. Braun Melsungen AG, Pfizer, and Fresenius Kabi, among others.
Buy Now

France Clinical Nutrition for Cancer Care Market Executive Summary
The France Clinical Nutrition for Cancer Care Market was valued at $154.8 Mn in 2023 and is predicted to grow at a CAGR of 4.04% from 2023 to 2030, to $204.3 Mn by 2030
Clinical nutrition for cancer care is a specialized field focusing on providing optimal nutritional support to patients throughout their cancer journey. It goes beyond simply “eating healthy” and involves a personalized approach to address the unique nutritional needs of each patient. A registered dietitian or other qualified healthcare professional usually assess the patient’s nutritional status. Based on the assessment, a personalized plan is created to meet the patient’s individual needs. Nutritional deficiencies are prevalent among cancer patients due to treatment side effects, altered metabolism, and decreased appetite. Clinical nutrition interventions play a crucial role in improving treatment tolerance as adequate nutrition can help patients tolerate chemotherapy and radiation therapy better, potentially reducing side effects and allowing for completion of treatment plans. Also, it maintains strength and immunity as proper nutrition helps patients maintain muscle mass, fight infections, and recover faster from surgery. Lastly, it enhances the quality of life since nutritional support can improve fatigue, appetite, and overall well-being, leading to a better quality of life during cancer treatment.
Advancements in medical nutrition therapy have led to the development of specialized oral nutritional supplements (ONS) and enteral feeding formulas. These products provide concentrated nutrients and can be crucial for patients struggling to meet their nutritional needs through regular diet alone. Parenteral nutrition remains an option for patients unable to tolerate oral or enteral feeding. Overall, clinical nutrition for cancer care plays a vital role in optimizing patient outcomes by ensuring they receive the necessary nutrients to support their body throughout treatment.
The France Clinical Nutrition for Cancer Care Market is driven by significant factors such as the increasing prevalence of cancer, increased awareness and education, and increasing healthcare expenditure. However, complexity of cancer care, reduced patient compliance, and stringent regulatory guidelines and approval process restrict the growth and potential of the market.
The leading players of the France Clinical Nutrition for Cancer Care Market are Danone Nutricia, Laboratories Lactalis Nutrition Santé (LNS), Nestlé Health Science, Abbott Nutrition, B. Braun Melsungen AG, Pfizer, and Fresenius Kabi, among others. Danone Nutricia, part of the Danone Group, is a global leader in specialized nutrition, focusing on infant nutrition, medical nutrition, and advanced medical nutrition. Notable products include Fortimel, Nutrison, and Neocate, which are designed for various clinical needs, including oncology nutrition.
Market Dynamics
Market Growth Drivers
Increasing Prevalence of Cancer: A major driver for this market is the increasing burden of cancer and the high mortality rates associated with it. As the population ages and lifestyle risks like smoking persist, the number of new cancer cases diagnosed each year continues to rise. In 2023, the number of new cases of cancer was estimated to be 4,33,136, of which 57% occurring in men. Both genders considered, the most frequent cancers are: breast cancer (61,214 new cases), prostate cancer (59,885 new cases) and lung cancer (52,777 new cases). This translates to a larger patient population requiring specialized nutritional support during treatment, which in turn drives the Clinical Nutrition for Cancer Care Market further.
Increased awareness and education: Increased awareness among healthcare providers, patients, and caregivers about the critical role of nutrition in cancer care drives demand for clinical nutrition products. Training programs and continuous education for oncologists, dietitians, and nurses on the benefits of clinical nutrition ensure that these professionals recommend and administer appropriate nutritional interventions to their patients. Increased awareness and education significantly contribute to the growth of the clinical nutrition market for cancer care by empowering patients, informing healthcare professionals, influencing public health policies, and driving research and product development. As more stakeholders recognize the critical role of nutrition in cancer treatment and recovery, the demand for clinical nutrition products continues to rise, expanding the market and improving patient outcomes.
Increasing Healthcare Expenditure: Higher healthcare expenditure allows for greater investment in R&D of advanced cancer treatments and nutritional products. This includes creating specialized formulas tailored to the needs of cancer patients, improving the efficacy and availability of clinical nutrition solutions. With more funding, healthcare providers can offer the latest cancer therapies, which often necessitate comprehensive nutritional support to manage side effects and enhance patient recovery. Increased spending leads to the development and modernization of hospitals and cancer treatment centres, equipped with the necessary infrastructure to provide comprehensive cancer care, including clinical nutrition services. Expanded healthcare budgets support the growth of homecare services, which include the delivery of clinical nutrition to cancer patients at home, making it more convenient and accessible. Thus, increased healthcare expenditure leads to the growth of France Clinical Nutrition for Cancer Care Market.
Market Restraints
Complexity of Cancer Care: Cancer care often involves a multidisciplinary team of oncologists, surgeons, radiologists, nurses, and dietitians. Coordinating the integration of clinical nutrition into comprehensive care plans can be complex and sometimes overlooked. Cancer patients have highly individualized nutritional needs based on their type of cancer, treatment regimen, and overall health. Creating and delivering personalized nutrition plans can be complex and resource-intensive. Also, side effects of cancer treatments can affect patients’ ability to tolerate certain nutritional products, leading to non-compliance or suboptimal nutrition. The complexity of managing multiple aspects of cancer care can lead to low patient compliance with nutritional recommendations, especially if patients are overwhelmed by their treatment regimen. Thus, the complexity of cancer care introduces several challenges that can reduce the market growth of clinical nutrition for cancer care.
Reduced Patient Compliance: Patient compliance is crucial for the effectiveness of nutritional interventions in cancer care. When patient compliance is low, it can significantly reduce the market of clinical nutrition for cancer care in several ways. When patients do not adhere to prescribed nutritional regimens, the expected therapeutic benefits are diminished. This can lead to poorer health outcomes, undermining the perceived value and efficacy of clinical nutrition products. If clinical nutrition products are perceived as ineffective due to non-compliance, healthcare providers may be less likely to recommend them, and patients may be less likely to use them, reducing overall demand. Non-compliance can lead to negative patient experiences if they do not see the expected improvements in their condition. This can result in dissatisfaction and a lack of trust in clinical nutrition products. Negative experiences can spread through word of mouth or online reviews, discouraging other patients and caregivers from using clinical nutrition products.
Stringent Regulatory Guidelines and Approval Process: The process for gaining approval from the regulatory authority of France can be very length and complex, often taking several years. This delay can slow the introduction of new clinical nutrition products to the market, hindering the ability to meet current patient needs promptly. Next, the high cost and risk associated with meeting regulatory standards can make companies more conservative in their innovation efforts. They might focus on modifying existing products rather than developing entirely new ones, limiting the variety and potential effectiveness of clinical nutrition products available to cancer patients. For niche products, particularly those targeting rare conditions or specific cancer-related nutritional needs, the potential return on investment might not justify the high costs of regulatory compliance, leading to fewer products being developed. While stringent regulatory approval and guidelines are essential for ensuring the safety and efficacy of clinical nutrition products, they can also act as significant barriers to market growth. These regulations can lead to longer time-to-market, higher costs, reduced innovation, and limited competition, all of which can decrease the availability and affordability of clinical nutrition products for cancer care.
Regulatory Landscape and Reimbursement Scenario
The main regulatory body for pharmaceuticals in France is the National Agency for the Safety of Medicines and Health Products (ANSM, Agence nationale de sécurité du médicament et des produits de santé in French). The ANSM is a government organization under the Ministry of Health which ensures the security of health products and facilitates access to innovative therapeutics. The ANSM guarantees the safety, efficacy, accessibility, and appropriate usage of health goods that are sold in France through evaluation, knowledge, and monitoring protocols.
The pharmaceutical companies must submit a completed MAA (Marketing Authorization Application) which can be used for drugs meant for the France market (National Procedure) or for the drugs intended for commercialization throughout the European Union (EU) through the EMA (European Medicines Agency). Through the EMA, products can be authorized through the National Procedure, the Centralised Procedure (CP), Decentralised Procedure (DCP) or Mutual Recognition Procedure (MRP). In this case, ANSM acts as a national competent authority (NCA) within the EMA framework. The ANSM then issues a final decision of either approval, conditional approval or refusal after conducting a review and evaluation of the MAA based on safety, efficacy, quality, and risk-benefit ratio.
The National Union of Health Insurance Funds of France (UNCAM, Union Nationale des Caisses d'Assurance Maladie) is the organization which is responsible for France’s public health insurance system. The UNCAM manages the several social security health insurance funds in charge of reimbursing healthcare costs for French nationals and residents. The French Health High Authority evaluates a medicine’s Medical Benefit (Service Médical Rendu, SMR) considering the severity of disease, safety and clinical efficacy, therapeutic innovation. The Committee on Economic Products for Health (CEPS) negotiates with pharmaceutical firms on the cost of medications. Lastly, the Ministry of Health, based on the recommendations of HAS and CEPS, renders the ultimate decision about reimbursement.
Competitive Landscape
Key Players
Here are some of the major key players in the France Clinical Nutrition for Cancer Care Market:
- Danone Nutricia
- Laboratories Lactalis Nutrition Santé (LNS)
- Nestlé Health Science
- Abbott Nutrition
- Pfizer
- B. Braun Melsungen AG
- Fresenius Kabi
- Danone Nutrica
- Baxter International Inc.
- Victus Inc.
- Mead Johnson Nutrition
- Aymes
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Clinical Nutrition for Cancer Care Market Segmentation
By Type
- Oral Nutrition
- Parenteral Nutrition
- Enteral Feeding Formulas
By Cancer Type
- Head & Neck Cancer
- Stomach & Gastrointestinal Cancer
- Blood Cancer
- Breast Cancer
- Lung Cancer
- Others
By Age Group
- Adult
- Paediatric
By Sales Channel
- Offline
- Online
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































