France Breast Pump Market Report
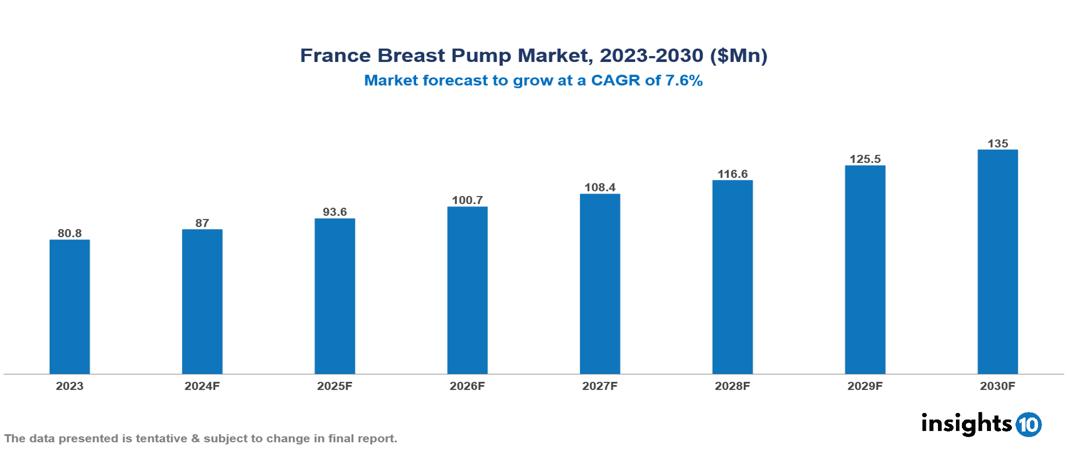
The France Breast Pump Market was valued at $80.8 Mn in 2023 and is predicted to grow at a CAGR of 7.6% from 2023 to 2030, to $135 Mn by 2030. The key drivers of the market include the rising female workforce, technological advancements, and growing consumer awareness. The prominent players in the France Breast Pump Market are Spectra Baby, Playtex, Ameda, Avent, Evenflo, and Pigeon, among others.
Buy Now

France Breast Pump Market Executive Summary
The France Breast Pump Market is at around $80.8 Mn in 2023 and is projected to reach $135 Mn in 2030, exhibiting a CAGR of 7.6% during the forecast period.
A breast pump is a mechanical device that lactating women use to extract milk from their breasts. They are available in two forms; they can be manual devices powered by hand or foot movements or automatic devices powered by electricity. Breast pumps are available in a variety of styles to meet the demands of moms. Hand-operated manual pumps are lightweight and silent, making them ideal for sporadic usage. Electric pumps are often used on a regular basis because of their higher efficiency and ability to run on mains or batteries. There are several uses for breast pumps. Many parents use them to continue breastfeeding after they return to work. They express their milk at work, which is later bottle-fed to their child by a caregiver. A breast pump may be also used to address a range of challenges parents may encounter during breastfeeding, including difficulties latching, separation from an infant in intensive care, feeding an infant who cannot extract sufficient milk itself from the breast, to avoid passing medication through breast milk to the baby, or to relieve engorgement, which is a painful condition whereby the breasts are overfull.
The France Breast Pump Market is driven by significant factors such as the rising female workforce, technological advancements, and growing consumer awareness. However, stringent regulations, physical discomfort, and negative perceptions of breast pumps restrict the growth and potential of the market.
The major players in the France Breast Pump Market are Spectra Baby, Playtex, Ameda, Avent, Evenflo, and Pigeon, among others. Evenflo Feeding provides a range of breast pumps designed for comfort and efficiency. Their pumps are user-friendly and designed to support a mother's breastfeeding journey.
Market Dynamics
Market Growth Drivers
Rising Female Workforce: In 2023, France's female labor force participation rate was 52.8%, compared to 60.1% for men. The increasing number of working women has driven demand for breast pumps, enabling mothers to express milk while at work, thus supporting breastfeeding. Enhanced healthcare infrastructure and government initiatives further bolster this market growth, making the expanding female workforce a key driver of breast pump demand in France.
Technological Advancements: Contemporary breast pumps include features like adjustable suction levels, digital displays, and memory functions to offer a customized pumping experience. The introduction of wearable and hands-free designs has enhanced convenience and discretion. Furthermore, smartphone connectivity enables tracking of pumping sessions and milk production. These advancements have significantly simplified and improved the breastfeeding experience for working mothers, positively impacting the market growth.
Growing Consumer Awareness: Factors such as educational campaigns, social media advocacy, and healthcare professionals promoting breastfeeding have contributed to this awareness. As more women recognize the benefits of breast pumps for maintaining breastfeeding while working or away from their babies, demand continues to rise. Innovations in technology, ease of use, and hygiene features further enhance consumer interest in breast pumps. Thus, the Breast Pump Market is witnessing growth due to heightened consumer awareness.
Market Restraints
Stringent Regulations: To ensure the quality, safety, and effectiveness of breast pumps, the French regulatory agency, ANSM, imposes stringent standards on medical devices. Complying with these regulations demands extensive testing, certification, and documentation, which can be costly and time-consuming for manufacturers. Navigating different regulatory frameworks in international markets adds further complexity. This regulatory burden can hinder smaller companies from entering the market, delay the introduction of new products, and increase costs for consumers, ultimately restricting market growth.
Physical Discomfort: Physical discomfort while using a breast pump is a common issue that many mothers experience due to poorly fitting flanges or incorrect pump usage. Manual pumps also lead to pain during and after continuous pumping sessions. A study stated that comfort is a critical factor in pump selection, with 68% of mothers citing pain as a concern. This discomfort can lead to negative perceptions of breast pumps, resulting in decreased demand and limiting market expansion.
Negative Perceptions of Breast Pumps: Breastfeeding mothers who use breast pumps are likely to face social stigma and negative perceptions. This stigmatization can create a non-supportive environment for breast pumping, which ultimately decreases the demand for breast pumps. Consequently, there is a slow growth of the Breast Pump Market due to a lesser demand.
Regulatory Landscape and Reimbursement Scenario
The main regulatory body for pharmaceuticals in France is the National Agency for the Safety of Medicines and Health Products (ANSM, Agence nationale de sécurité du médicament et des produits de santé in French). The ANSM is a government organization under the Ministry of Health that ensures the security of health products and facilitates access to innovative therapeutics. The ANSM guarantees the safety, efficacy, accessibility, and appropriate usage of health goods that are sold in France through evaluation, knowledge, and monitoring protocols.
The pharmaceutical companies must submit a completed MAA (Marketing Authorization Application) which can be used for drugs meant for the France market (National Procedure) or for the drugs intended for commercialization throughout the European Union (EU) through the EMA (European Medicines Agency). Through the EMA, products can be authorized through the National Procedure, the Centralised Procedure (CP), the Decentralised Procedure (DCP), or the Mutual Recognition Procedure (MRP). In this case, ANSM acts as a national competent authority (NCA) within the EMA framework. The ANSM then issues a final decision of either approval, conditional approval, or refusal after conducting a review and evaluation of the MAA based on safety, efficacy, quality, and risk-benefit ratio. The Medical Device and Technology Evaluation Committee (CNEDiMTS) is the agency that examines medical devices and classifies them based on their level of risk into the following four classes: Class I for low degree of risk, Class IIa for moderate degree of risk, Class IIa for high potential of risk, and Class III for very serious potential of risk.
The National Union of Health Insurance Funds of France (UNCAM, Union Nationale des Caisses d'Assurance Maladie) is the organization that is responsible for France’s public health insurance system. The UNCAM manages several social security health insurance funds in charge of reimbursing healthcare costs for French nationals and residents. The French Health High Authority evaluates a medicine’s Medical Benefit (Service Médical Rendu, SMR) considering the severity of the disease, safety and clinical efficacy, and therapeutic innovation. The Committee on Economic Products for Health (CEPS) negotiates with pharmaceutical firms on the cost of medications. Lastly, the Ministry of Health, based on the recommendations of HAS and CEPS, renders the ultimate decision about reimbursement.
Competitive Landscape
Key Players
Here are some of the major key players in the France Breast Pump Market:
- Spectra Baby
- Playtex
- Ameda
- Avent
- Evenflo
- Pigeon
- Hygeia
- Willow
- Perifit
- Kimetech GmbH
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Breast Pump Market Segmentation
By Product Type
- Manual pumps
- Battery-powered pumps
- Electric pumps
By Pump System
- Open System
- Closed System
By Pumping Type
- Single
- Double
By Distribution Channel
- Hospital Pharmacies
- Retail Stores
- Online Stores
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































