France Biosimilars Market Analysis
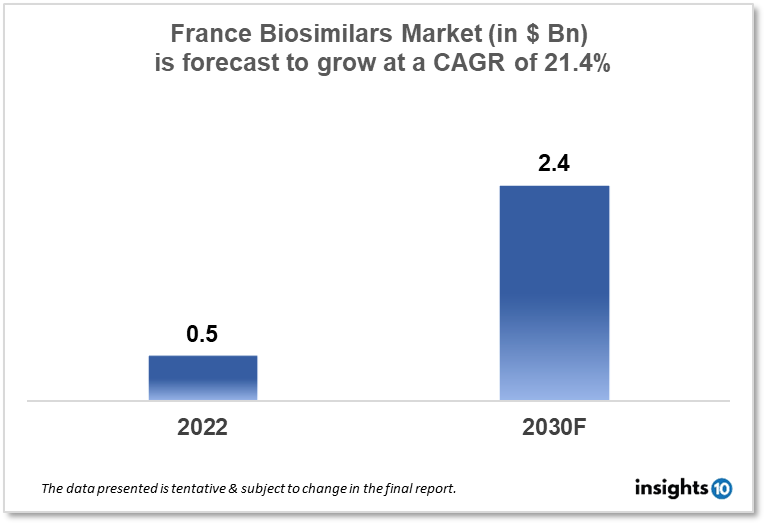
France's biosimilars market size was valued at $0.5 Bn in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 21.4% from 2022 to 2030 and will reach $2.4 Bn in 2030. The market is segmented by product type and indication type. The France Biosimilars market will grow with the increased incidence of chronic illnesses, rising healthcare expenses, and the demand for affordable biologic medicine substitutes. The key market players are Sanofi, Pfizer, Biogen, Mylan, Amgen, and others.
Buy Now

France Biosimilars Market Executive Summary
The France Biosimilars market size was valued at $0.5 Bn in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 21.4% from 2022 to 2030 and will reach $2.4 Bn in 2030. With substantial growth potential in the upcoming years, biosimilars are becoming a major portion of the French pharmaceutical business. With several initiatives in place to stimulate the use of biosimilars, France has been one of the leading European nations in this area.
The desire to offer more inexpensive alternatives to biologic medications, which can be prohibitively expensive for many patients and healthcare systems, is what essentially drives the French biosimilar business. When compared to their reference goods, biosimilars are less expensive, and the French government has taken a number of steps to encourage the adoption of these medicines.
The "Plan de Développement des Médicaments Génériques et Biosimilaires" (Generic and Biosimilar Drug Development Plan), which was introduced in 2015, is one of the major projects. By fostering the development of these medications and encouraging healthcare professionals to utilize them, this initiative seeks to enhance the availability of biosimilars in France.
Overall, it is anticipated that the French biosimilar market would expand over the coming years due to reasons such as the increased incidence of chronic diseases, rising healthcare expenses, and the demand for more economical therapies. The market is anticipated to increase significantly as a result of the French government's attempts to promote biosimilars.
Market Dynamics
Market Growth Drivers
The increased incidence of chronic illnesses, rising healthcare expenses, and the demand for affordable biologic medicine substitutes are some of the drivers driving the French biosimilar industry. Because they are significantly less expensive than their reference products, biosimilars are becoming more and more well-liked by payers and healthcare providers.
Additionally, the French government has taken steps to encourage the use of biosimilars, including establishing a national platform to track their usage and providing financial incentives to hospitals that employ them. The biosimilar market in France is anticipated to increase as a result of these measures.
Market Restraints
Although the French market for biosimilars has room for expansion, there are a number of obstacles preventing it. High regulatory requirements are one of the main obstacles since they might make developing and releasing biosimilar goods more expensive and time-consuming.
The low understanding and acceptability of healthcare professionals and patients may potentially be a deterrent to the use of biosimilars. Due to concerns about effectiveness and safety, patients may be reluctant to convert to biosimilars, and healthcare professionals may not have the appropriate skills and education to recommend biosimilars.
Competitive Landscape
Key Players
- Pfizer (USA)
- Biogen (USA)
- Sandoz (Switzerland)
- Mylan (USA)
- Amgen (USA)
- Fresenius Kabi (Germany)
- Teva (Israel)
- Sanofi (France)
- Merck (USA)
- Boehringer Ingelheim (Germany)
Healthcare Policies and Regulatory Landscape
The National Agency for the Safety of Medicines and Health Products (ANSM) oversees biosimilar regulation in France. For the approval of biosimilars, the ANSM adheres to the standards established by the European Medicines Agency (EMA).
The National Union of Health Insurance Funds (UNCAM) and the Transparency Commission are in charge of managing biosimilar reimbursement in France. The Transparency Commission assesses the clinical and financial viability of new medications, including biosimilars, to determine whether the French national health insurance system should pay for them.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Biosimilars Market Segmentation
By Product
The monoclonal antibodies, insulin, granulocyte colony-stimulating factor, erythropoietin, recombinant human growth hormone, etanercept, follitropin, teriparatide, interferons, enoxaparin sodium, glucagon, and calcitonin are among the product categories that make up the biosimilars market. Monoclonal antibodies held a sizable portion of the market in 2020. The market is being driven by elements including the widespread use of monoclonal antibodies in the treatment of autoimmune diseases, cancer, and osteoporosis, as well as the affordability of such treatments.
- Monoclonal Antibodies
- Infliximab
- Trastuzumab
- Rituximab
- Adalimumab
- Other monoclonal antibodies (bevacizumab, cetuximab, ranibizumab, denosumab, and eculizumab)
- Insulin
- Granulocyte Colony-Stimulating Factor
- Erythropoietin
- Recombinant Human Growth Hormone
- Etanercept
- Follitropin
- Teriparatide
- Interferons
- Enoxaparin Sodium
- Glucagon
- Calcitonin
By Indication
The biosimilars market is divided into oncology, autoimmune and inflammatory diseases, chronic illnesses, blood disorders, growth hormone insufficiency, infectious diseases, and other indications based on the indication (infertility, hypoglycemia, myocardial infarction, postmenopausal osteoporosis, chronic kidney failure, and ophthalmic diseases). The market's largest sector in 2020 will be oncology. This market is expanding as a result of elements like the reduced cost of biosimilars compared to novel biologics and the increased incidence and prevalence of cancer.
- Oncology
- Inflammatory & Autoimmune Disorders
- Chronic Diseases
- Blood Disorders
- Growth Hormone Deficiency
- Infectious Diseases
- Other Indications (infertility, hypoglycemia, postmenopausal osteoporosis, chronic kidney failure, and ophthalmic diseases)
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































