France Biosensors Market Analysis
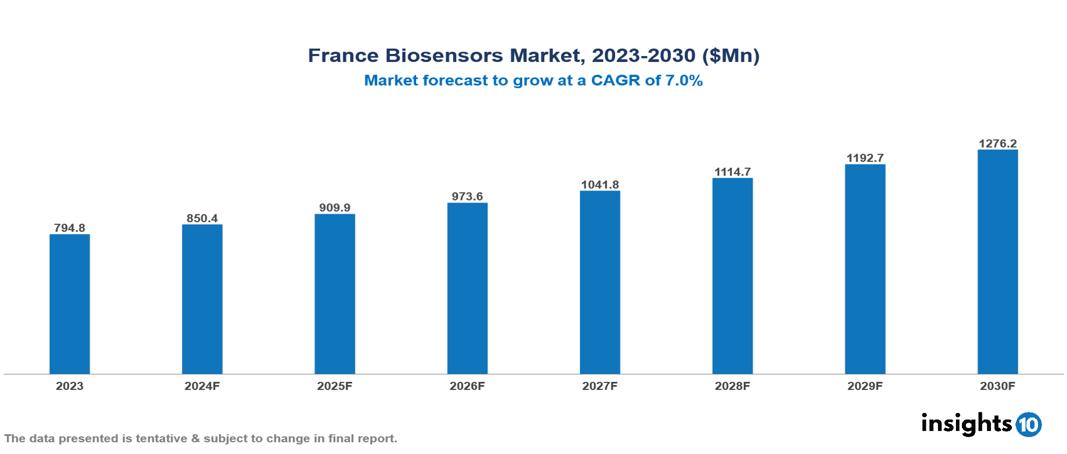
The France Biosensors Market was valued at $794.8 Mn in 2023 and is predicted to grow at a CAGR of 7.0% from 2023 to 2030, to $1276.2 Mn by 2030. The key drivers of the market include increasing burden of chronic diseases, technological advancements, and growing demand for Point-of-Care (POC) testing. The prominent players of the France Biosensors Market are Villard Medical, Bio-Rad International, Masimo Corporation, Meridian Bioscience, and Biosensors International, among others.
Buy Now

France Biosensors Market Executive Summary
The France Biosensors market is at around $794.8 Mn in 2023 and is projected to reach $1276.2 Mn in 2030, exhibiting a CAGR of 7% during the forecast period.
Biosensors are devices that convert a biological response into a measurable electrical signal. They generate signals which are proportional to the concentration of the analyte in the reaction. Applications of biosensors include disease monitoring, drug discovery and development, and the identification of contaminants, pathogen-causing microorganisms, and disease-indicating markers in physiological fluids such as blood, urine, saliva, and sweat. A biosensor consists of components, namely the analyte, bioreceptor, transducer, electronics, and the display. Analyte is the target molecule which is to be detected in a sample. For instance, an analyte could be glucose which is to be measured in a diabetic patient. A bioreceptor is molecule that specifically recognises the analyte. Examples of bioreceptors include enzymes, cells, aptamers, DNA, and antibodies. Bio-recognition is the process of generating a signal in the form light, heat, pH, charge or mass shift when the bioreceptor and analyte interact. Another important component of biosensors is the transducer which functions to convert one form of energy into another, more specifically it converts the biological signal from the bioreceptor into an electrical signal in a process known as signalisation. Transducers can be electrochemical, optical, or piezoelectric. Electronics is the part of a biosensor that processes the transduced signal and prepares it for display. Lastly, the display consists of a user interpretation system such as the LCD of a computer or a direct printer which generates an output based on the requirements of the end user.
Chronic diseases are a major concern for the French healthcare system, impacting both public health and economic well-being and eventually leading to morbidity and mortality. The France Biosensors Market is thus driven by significant factors such as the increasing burden of chronic diseases, technological advancements, and growing demand for Point-of-Care (POC) testing. However, stringent regulatory requirements, technical challenges, and data management issues restrict the growth and potential of the market.
The major players of the France Biosensors Market are Villard Medical, Bio-Rad International, Masimo Corporation, Meridian Bioscience, and Biosensors International, among others.
Market Dynamics
Market Growth Drivers
Increasing Burden of Chronic Diseases: The global burden of chronic conditions such as hypertension, obesity, and auto-immune disorders is increasing day by day. For instance, the age-standardised rate for all cancers for men and women combined was 339 in the year 2022 according to the World Cancer Research Fund International. The healthcare treatment takes a longer period of time which can lead to lower quality of life for patients. However, consistent monitoring for effective management is important which is conveniently achieved through the use of biosensors. Biosensors reduce the need for invasive treatments and frequent hospital visits by enabling patients to monitor key health metrics at home. This real-time monitoring capability supports better chronic disease management, early diagnosis, and treatment, preventing complications and improving long-term health outcomes, thereby driving the growth of the biosensors market.
Technological Advancements: Advancements in biosensor technology are rapidly evolving. Innovative uses of fluorescence tagging and nanomaterials like graphene and carbon nanotubes have led to enhanced sensitivity and detection limits in biosensors. The incorporation of aptamers and nucleotides as recognition elements is advancing new biosensor technologies. Miniaturization of biosensors has become feasible due to breakthroughs in nanotechnology, materials science, and microfabrication, resulting in devices with superior sensitivity, specificity, and faster reaction times. Wearable biosensors now utilize biocompatible materials, reducing rejection risks and ensuring patient comfort and compliance. Furthermore, the combination of biosensors with AI and ML algorithms improves data analysis accuracy and efficiency, enabling personalized healthcare strategies and early disease detection. These technological advancements are boosting the performance of biosensors, leading to market growth.
Growing Demand for Point-Of-Care (POC) Testing: Increasing demand for Point-Of-Care (POC) testing is a major factor in the growth of the biosensors market for several reasons. Biosensors are ideally suited for POC testing because they offer benefits such as portability, ease of use, and rapid results that conventional lab-based methods often lack. Additionally, POC testing with biosensors makes healthcare services more accessible, particularly in remote areas or for patients with limited mobility. The COVID-19 pandemic underscored the importance of POC testing, with biosensors playing a key role in overcoming the crisis. Thus, the rising demand for POC testing is driving the growth of the biosensors market.
Market Restraints
Stringent Regulatory Requirements: Biosensors, especially those designed for medical use, can significantly impact people's health. Companies in this field must comply with GDPR and HIPAA regulations. Medical devices undergo rigorous testing by country-specific regulatory bodies to ensure accuracy, safety, and to assess risks like data security and biocompatibility. Although strict guidelines protect patients and build market trust, the lengthy and expensive approval process can delay the introduction of innovative biosensors. This can limit innovation and restrict patient access. Furthermore, the high costs of meeting regulatory requirements result in increased biosensor prices, preventing the market from fully expanding.
Technical Challenges: The healthcare sector is being revolutionized by continuous biosensor advancements, yet technical challenges still exist. For accurate diagnoses, biosensor sensitivity, specificity, and accuracy must be constantly improved. One major challenge is the biosensor's ability to distinguish between the target analyte and other sample constituents. Additionally, detecting low concentrations of analytes is crucial for early disease diagnosis and environmental monitoring, but achieving such high sensitivity in complex biological samples is difficult. Ensuring reproducibility of biosensor results is also crucial but challenging. These issues slow down the growth of the biosensors market.
Data Management Issues: Biosensors generate copious amounts of health data, which makes it imperative to store, organize, and analyse the data efficiently. This requires sophisticated data management solutions and a strong data management infrastructure, which can be expensive and challenging to maintain. Accuracy and precision are necessary for the data gathered by biosensors in order to facilitate efficient data processing and decision-making. Poor data quality can result in damaging decisions and inaccurate conclusions that can be hazardous to patients' health, especially in the medical industry. Furthermore, because different biosensors have different formats, standards, and procedures, integrating the data from these biosensors can be difficult if many biosensors are used. As a result, this compatibility may make it more difficult for departments to share data and work together. In general, the various problems with data management can hinder the potential growth of the market.
Regulatory Landscape and Reimbursement Scenario
The main regulatory body for pharmaceuticals in France is the National Agency for the Safety of Medicines and Health Products (ANSM, Agence nationale de sécurité du médicament et des produits de santé in French). The ANSM is a government organization under the Ministry of Health which ensures the security of health products and facilitates access to innovative therapeutics. The ANSM guarantees the safety, efficacy, accessibility, and appropriate usage of health goods that are sold in France through evaluation, knowledge, and monitoring protocols.
The pharmaceutical companies must submit a completed MAA (Marketing Authorization Application) which can be used for drugs meant for the France market (National Procedure) or for the drugs intended for commercialization throughout the European Union (EU) through the EMA (European Medicines Agency). Through the EMA, products can be authorized through the National Procedure, the Centralised Procedure (CP), Decentralised Procedure (DCP) or Mutual Recognition Procedure (MRP). In this case, ANSM acts as a national competent authority (NCA) within the EMA framework. The ANSM then issues a final decision of either approval, conditional approval or refusal after conducting a review and evaluation of the MAA based on safety, efficacy, quality, and risk-benefit ratio. The Medical Device and Technology Evaluation Committee (CNEDiMTS) is the agency which examines the medical devices and classifies them based on their level of risk into the following four classes: Class I for low degree of risk, Class IIa for moderate degree of risk, Class IIa for high potential of risk, and Class III for very serious potential of risk.
The National Union of Health Insurance Funds of France (UNCAM, Union Nationale des Caisses d'Assurance Maladie) is the organization which is responsible for France’s public health insurance system. The UNCAM manages the several social security health insurance funds in charge of reimbursing healthcare costs for French nationals and residents. The French Health High Authority evaluates a medicine’s Medical Benefit (Service Médical Rendu, SMR) considering the severity of disease, safety and clinical efficacy, therapeutic innovation. The Committee on Economic Products for Health (CEPS) negotiates with pharmaceutical firms on the cost of medications. Lastly, the Ministry of Health, based on the recommendations of HAS and CEPS, renders the ultimate decision about reimbursement.
Competitive Landscape
Key Players
Here are some of the major key players in the France Biosensors Market:
- Villard Medical
- Bio-Rad International
- Masimo Corporation
- Meridian Bioscience
- Biosensors International
- Nix Biosensors
- Abbott Laboratories
- Medtronic
- Siemens Healthcare
- F. Hoffmann-La Roche
1. Executive Summary
1.1 Device Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Regulatory Landscape for Medical Device
1.6 Health Insurance Coverage in Country
1.7 Type of Medical Device
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Biosensors Market Segmentation
By Technology
- Electrochemical Biosensors
- Optical Biosensors
- Piezoelectric Biosensors
- Thermal Biosensors
- Nanomechanical Biosensors
By Product
- Wearable Biosensors
- Non-wearable Biosensors
By Application
- Medical Diagnostics
- Food Safety
- Environmental Monitoring
- Agriculture and Bioreactor Monitoring
- Other
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































