France Bioinformatics Market Analysis
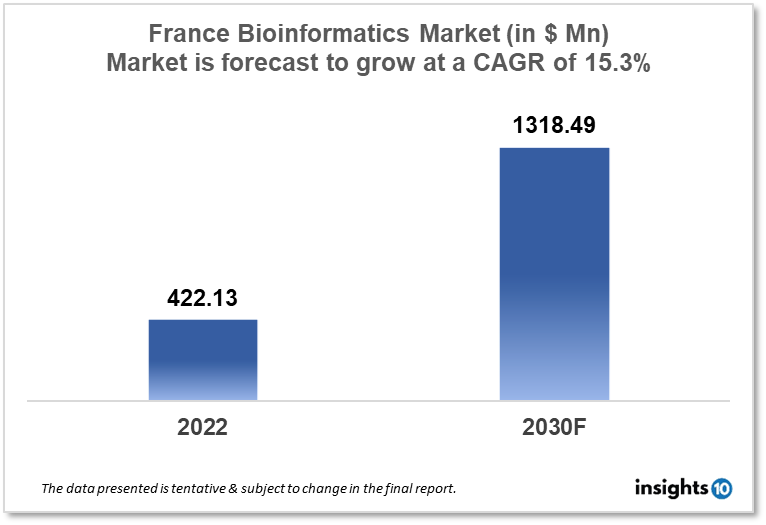
The France Bioinformatics market is projected to grow from $422.13 Mn in 2022 to $1,318.49 Mn by 2030, registering a CAGR of 15.30% during the forecast period of 2022 - 2030. The main factors driving the growth would be increasing demand for precision medicine, advancements in the area of genomics, transcriptomics and proteomics technologies and government support in the development of bioinformatics market. The market is segmented by technology and by application. Some of the major players include GenoScreen (FRA), BioMérieux (FRA), Hybrigenics Corporation (FRA), Illumina Inc., Agilent Technologies and IBM Life Sciences.
Buy Now

France Bioinformatics Market Executive Summary
The France Bioinformatics market is projected to grow from $422.13 Mn in 2022 to $1,318.49 Mn by 2030, registering a CAGR of 15.30% during the forecast period of 2022 - 2030. In 2020, France spent $227 Bn on healthcare or about $3,364 per person. France has long outperformed the EU norm in terms of health spending both as a percentage of GDP and per capita. Prior to 2020, health spending expanded at roughly the same rate as the economy, but it did so more quickly in reaction to the COVID-19 epidemic, which caused GDP to decline by 8%.
Bioinformatics is a multidisciplinary field of biology and information technology. Bioinformatics employs a variety of approaches, including data production, data warehousing, data mining, data management, and others. Utilized as integrated solutions, bioinformatics software and tools provide statistical approaches and algorithms for data processing for applications such as next-generation sequencing, modeling of genomic and proteomic structure, and three-dimensional drug discovery. In the global bioinformatics market, Europe is the second largest leader globally and the France bioinformatics market is considered to be one of the most developed markets in Europe.
Market Dynamics
Market Growth Drivers
The France bioinformatics market is expected to be driven by factors such as increasing demand for precision medicine, advancements in the area of genomics, transcriptomics, and proteomics technologies, and government support in the development of the bioinformatics market. Additionally, France has a national bioinformatics infrastructure, the French Institute of Bioinformatics (IFB) which supports the life sciences communities by deploying services, coordinating training, and carrying out creative advancements.
Market Restraints
The main factor limiting the growth of the bioinformatics market in France is the lack of knowledge about data management plans. As per the survey conducted by IFB, there is a huge necessity for spreading knowledge and training about data management plans for boosting the bioinformatics market.
Competitive Landscape
Key Players
- GenoScreen (FRA)
- BioMérieux (FRA)
- Hybrigenics Corporation (FRA)
- Illumina Inc.
- Agilent Technologies
- IBM Life Sciences
Notable Recent Deals
October 2022: Illumina Inc. partnered with GenoScreen to increase global access to genomic testing for multi-drug resistant tuberculosis. This partnership enabled global access to a package combining Illumina sequencing products and the GenoScreen Deeplex® Myc-TB assay, a targeted next-generation sequencing-based test for rapid and extensive detection of anti-TB drug resistance, for immediate treatment decision-making.
Healthcare Policies and Regulatory Landscape
The healthcare policies and regulations in France are governed by several laws and agencies. The French Data Protection Authority (CNIL) is responsible for the enforcement of data protection laws to ensure the security and privacy of personal data. The French Health Products Safety Agency (AFSSAPS) is for regulating health products’ safety and efficacy. The French National Institute for Health and Medical Research (INSERM) and The French National Agency for Research (ANR) are responsible for funding and supporting research in several fields including bioinformatics.
Reimbursement Scenario
The reimbursement policies in France are established by the French National Health Insurance (Assurance Maladie) and the French Health Products Safety Agency (AFSSAPS). The French national health insurance makes case-by-case decisions about reimbursement for bioinformatics goods and services, taking into account elements including the medical need, the efficacy and safety of the product or service, and the cost-effectiveness. The French national health insurance uses a Temporary Authorisation for Use (ATU) which permits coverage for innovative medical products and medications that are still being approved. Innovative Therapy Refunding Agreement (ATRi) provides a framework for the reimbursement of innovative therapies with a high price but considerable public health benefits.
1. Executive Summary
1.1 Service Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Healthcare Services Market in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Services
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Bioinformatics Market Segmentation
By Technology (Revenue, USD Billion):
- Knowledge Management Tools
- Bioinformatics Platforms
- Bioinformatics Services
By Application (Revenue, USD Billion):
- Metabolomics
- Molecular phylogenetics
- Transcriptomics
- Chemoinformatic & drug design
- Genomics
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































