France Artificial Intelligence (AI) in Diagnostics Market Analysis
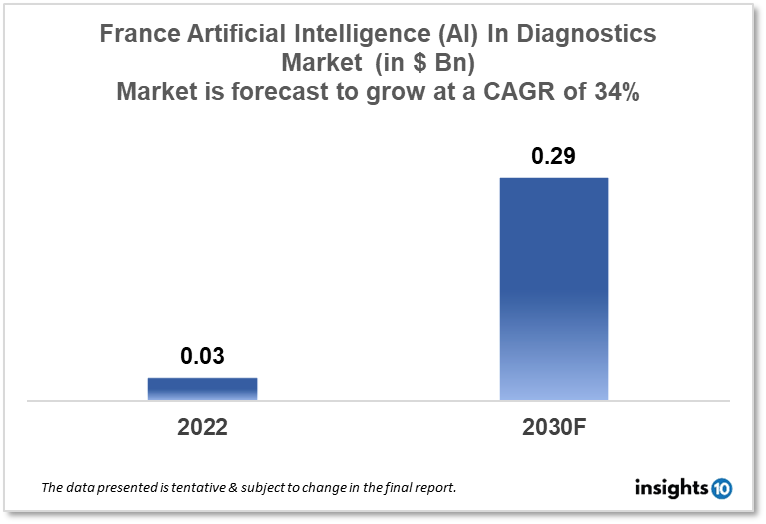
France's Artificial Intelligence (AI) in the Diagnostics market is projected to grow from $0.03 Bn in 2022 to $0.29 Bn by 2030, registering a CAGR of 34% during the forecast period of 2022 - 2030. The market will be driven by a rise in the adoption of AI technology due to supportive regulatory policies and rising investments by new startups in France. The market is segmented by component & by diagnosis. Some of the major players include Siemens Healthineers, Philips Healthcare & Owkin.
Buy Now

France Artificial Intelligence (AI) in Diagnostics Market Executive Summary
France's Artificial Intelligence (AI) in the Diagnostics market is projected to grow from $0.03 Bn in 2022 to $0.29 Bn by 2030, registering a CAGR of 34% during the forecast period of 2022 - 2030. France is the world's seventh-largest economy, with a GDP of about $2.94 Tn in 2021 and expected $2.63 Tn in 2022, and Europe's third-largest economy after Germany and the United Kingdom. According to WHO data published in 2020, 63,181 people died from coronary heart disease in France, accounting for 13.22% of all fatalities. France ranked 182 in the world with an age-adjusted death rate of 30.17 per 100,000 inhabitants. France is a model for medical imaging, with 8,885 radiodiagnosis and medical imaging specialists and roughly 33,500 electro-radiology manipulators.
In France, artificial intelligence-based diagnostic techniques and technologies are employed in a variety of medical professions, including radiology, pathology, cardiology, and dermatology. AI algorithms are being used to analyze diagnostic imaging in order to detect and diagnose a variety of medical disorders such as cancer, Alzheimer's disease, and skin cancer. AI is also being utilized to create prediction models and decision support systems that can assist doctors in making more accurate diagnoses and treatment decisions. A favorable legislative and policy framework, including rules and regulations created by the French National Authority for Health, supports the deployment of AI in diagnostics in France (HAS).
In July 2020, Medipath, which provides pathology services to over 170 hospitals and clinics throughout France, completed the integration of Ibex's Galen Prostate into its usual clinical practice. A highly accurate AI system examines prostate biopsies and raises alarms when inconsistencies with pathologists' initial diagnosis are discovered in Ibex's CE-marked solution.
Market Dynamics
Market Growth Drivers
Numerous French firms, both established and start-up, are working in AI in the diagnostics industry. These firms are working on a variety of AI-based diagnostic tools and technologies, such as software for computer-aided diagnosis, predictive analytics, and clinical decision support. The French government has also been supportive of the research and application of AI in healthcare, particularly in diagnostics. The French government announced the "AI for Health" program in 2019, with the goal of accelerating the development of AI technology for healthcare applications such as diagnostics. Such factors play a key role in enhancing patient outcomes and thereby contribute to the growth of the AI Diagnostics market.
Market Restraints
Data privacy and security are two major barriers to the deployment of AI in diagnostics in France. There is rising concern over the use of personal data in AI-based diagnostic tools, with requests for more regulation and monitoring to guarantee that patient data is utilized legally and responsibly. Another issue is the high implementation cost. Creating and deploying AI-based diagnostic tools and technologies necessitates substantial investment in hardware, software, and staff, which may be prohibitive for certain healthcare organizations, particularly smaller institutions.
Competitive Landscape
Key Players
- Siemens Healthineers
- Philips Healthcare
- GE Healthcare
- Biomodex (FRA)
- Owkin (FRA)
- InnovaFeed (FRA)
- Siview (FRA)
Notable Deals
- September 2022, SiVIEW, a leader in AI-driven visual diagnostics, has announced a €5.5 million capital raising with LBO France
- September 2022, Owkin, an artificial intelligence (AI) biotech firm, announced today the approval for use in Europe of two first-in-class quick, cheap AI-based diagnostic products targeted to enhance outcomes for patients with breast cancer and colorectal cancer
Healthcare Policies and Regulatory Landscape & Reimbursement Scenario
France has defined standards and laws for the use of AI in healthcare, including diagnostics, in terms of regulatory and reimbursement systems. The French National Authority for Health (HAS) has released recommendations on the use of AI-based diagnostic tools, as well as a legal framework for evaluating and approving these technologies.
In France, reimbursement for AI-based diagnostic tools and technology is also available through the national health insurance system. Nevertheless, reimbursement rates and rules may differ based on the technology and its intended application.
1. Executive Summary
1.1 Digital Health Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Digital Health Policy in Country
1.6 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Market Size (With Excel and Methodology)
2.2 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Artificial Intelligence (AI) in Diagnostics Market Segmentation
- By Component Outlook Type (Revenue, USD Billion):
- Software
- Hardware
- Services
- By Diagnosis Outlook Type (Revenue, USD Billion):
- Cardiology
- Oncology
- Pathology
- Radiology
- Chest and Lung
- Neurology
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.









































