France Anemia Therapeutics Market Analysis
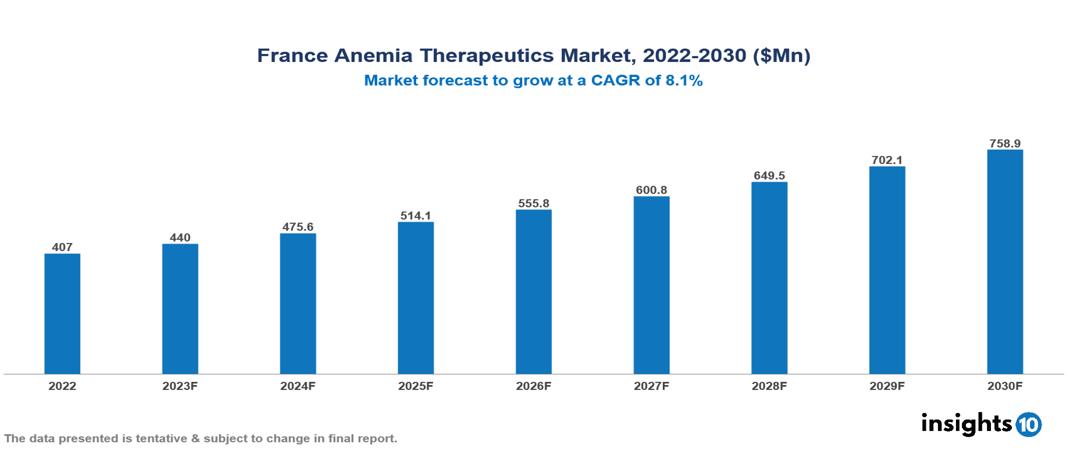
The France Anemia Therapeutics Market is anticipated to experience a growth from $407 Mn in 2022 to $759 Mn by 2030, with a CAGR of 8.10 % during the forecast period of 2022-2030. Higher prevalence owing to unhealthy lifestyles, initiatives to eliminate societal stigma associated with specific anemias, and improvements in technologies like as gene therapy, gene editing, and innovative medication delivery techniques are among the factors driving market growth. The France Anemia Therapeutics Market encompasses various players across different segments, including Sanofi, Amgen, Novartis, AstraZeneca, Spark Therapeutics, Bluebird Bio, Emmaus Medical, Mylan, Sandoz, Institut Mérieux etc, among various others.
Buy Now

France Anemia Therapeutics Market Analysis Executive Summary
The France Anemia Therapeutics Market is anticipated to experience a growth from $407 Mn in 2022 to $759 Mn by 2030, with a CAGR of 8.10 % during the forecast period of 2022-2030.
Anemia is a condition characterized by a decrease in the number of red blood cells or hemoglobin in the blood, causing fatigue, weakness, and pallor. It is divided into several types, including iron deficiency, vitamin B12 or B9 deficiency, chronic inflammatory sickness, chronic renal disease, and autoimmune hemolytic anemia. The most common kind of anemia in the world is iron deficiency anemia, which affects people of all ages, including babies, teens, and pregnant women. Treatment for iron deficiency anemia includes dietary advice as well as oral and intravenous iron supplements. Vitamin B12 or B9 deficiency anemia can be treated with iron or vitamin supplements; in severe cases, an infusion may be necessary. Chronic inflammatory disease anemia, chronic renal disease anemia, and autoimmune hemolytic anemia all require treatment for the underlying condition to alleviate symptoms. Sickle cell anemia and thalassemia are two types of inherited anemias with no recognized therapies. In contrast, iron-rich foods, including a well-balanced diet, can help prevent iron deficiency anemia.
Anemia affects around 4–8% of the French population, which equates to 2-4 million individuals. Anemia is more prevalent in women, particularly pregnant women and those of reproductive age, due to factors such as iron deficiency and monthly blood loss. Iron deficiency anemia is the most common form in France, followed by anemia caused by chronic illnesses such as renal disease and cancer. Higher prevalence owing to unhealthy lifestyles, initiatives to eliminate societal stigma associated with specific anemias, and improvements in technologies like gene therapy, gene editing, and innovative medication delivery techniques are among the factors driving market growth.
Sanofi holds a dominant position in the market, providing well-established medicines for a wide range of anemias across all sectors. Another major participant, Novartis, is increasing its contribution through gene therapy development for beta-thalassemia and sickle cell disease, which has the potential for large future income. Local companies, such as Mylan, make a lot of money selling generic iron supplements and other anemic remedies on the market.
Market Dynamics
Market Growth Drivers
Lifestyle Changes: Changes, such as an unhealthy diet, a lack of physical exercise, and access to healthcare, can all have an impact on the prevalence and management of anemia. Certain lifestyle variables that contribute to chronic diseases such as renal disease or cancer, which can lead to anemia as a subsequent result, may have an indirect impact on the demand for anemia therapies tailored to these disorders. Public health programs often promote healthy lifestyles and preventative measures, which can help drive market growth.
Social stigma: Some anemias are associated with social stigma, which can delay diagnosis and treatment. Addressing this stigma may enhance patient outcomes and boost demand for therapy. Cultural ideas and practices about blood and illness can shape disease perception and treatment-seeking behaviour. Understanding these elements is critical to successful communication and intervention.
Technological Push: Gene therapy, gene editing, and other cutting-edge technologies provide promising treatments for uncommon anemias, as well as possible future uses. Advancements in oral iron formulations, controlled-release capsules, and other delivery techniques promise enhanced compliance and patient experience. Emerging research on genetic susceptibility and biomarkers may pave the way for tailored treatment methods.
Market Restraints
Reliance on Established Players: While competition promotes innovation, severe rivalry may result in pricing pressures and industry consolidation, possibly restricting product diversity and patient options. Dependence on major pharmaceutical corporations for novel cures might limit market access for smaller businesses and impede diversification.
Economic and Pricing Challenges: Gene therapies and other novel treatments can be prohibitively expensive, raising economic problems for individuals and the healthcare system. This may impede patient access and market expansion. Government and commercial insurance plans may not cover the full cost of new medications, posing hurdles to market access and patient affordability. France's healthcare budget is constrained, potentially limiting the deployment of money to costly anemic therapies.
Inequalities in Access to Care: Unequal access to healthcare services and experts might hinder underprivileged groups from receiving accurate diagnosis and treatment for anemia. Insufficient public understanding of anemia symptoms and treatment choices might result in delayed diagnosis and missed possibilities for early management.
Healthcare Policies and Regulatory Landscape
France has always been at the forefront of healthcare policy, emphasizing universal access to quality medical treatment. The country's healthcare system is mostly public, with the government significantly involved in funding and regulating healthcare services. The drug regulating authority, the Agence nationale de sécurité du médicament et des produits de santé (ANSM), is an important part of this system since it ensures the safety and efficacy of medicines in the French market. ANSM thoroughly assesses medications before approval and monitors their post-marketing performance to ensure continuous safety. This regulatory organization not only protects public health, but it also helps to improve the overall efficiency of the healthcare system by ensuring that patients have access to safe and effective treatments. Furthermore, it actively engages in the development of healthcare policy, contributing professional advice to government choices. France's commitment to a strong regulatory system reflects its determination to sustaining high healthcare standards and emphasizes the necessity of a watchful drug regulatory body in defining and protecting public health.
Competitive Landscape
Key Players:
- Sanofi
- Amgen
- Novartis
- AstraZeneca
- Spark Therapeutics
- bluebird bio
- Emmaus Medical
- Mylan
- Sandoz
- Institut Mérieux
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
France Anemia Therapeutics Market Segmentation
By Type of Disease
- Iron Deficiency Anemia
- Megaloblastic Anemia
- Pernicious Anemia
- Hemorrhagic Anemia
- Hemolytic Anemia
- Sickle Cell Anemia
By Population
- Pediatrics
- Adults
- Geriatrics
By Therapy Type
- Oral Iron Therapy
- Parenteral Iron Therapy
- Red Blood Cell Transplantation
- Others
By Distribution Channel
- Hospital Pharmacies
- Drug Stores & Retail Pharmacies
- Online Pharmacies
By End User
- In-Patient Centres
- Out-Patient Speciality Clinics
- Homecare
- Others
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.