Europe Alzheimer’s Disease Drugs Market Analysis
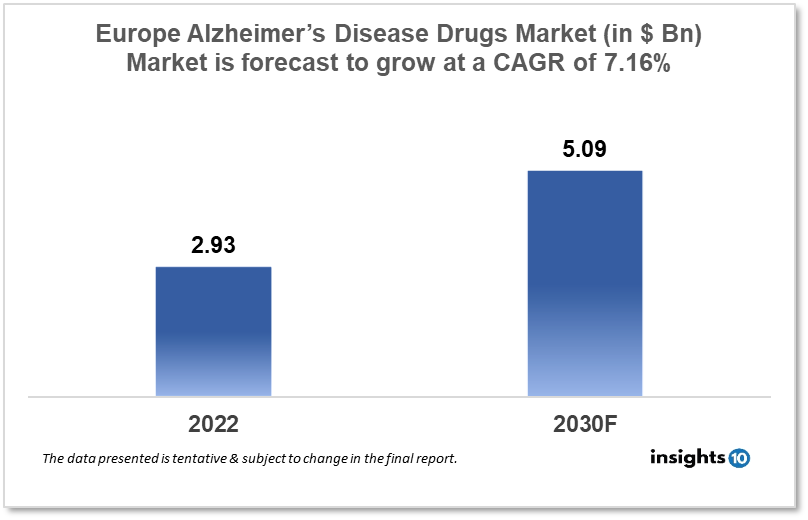
Europe's Alzheimer’s disease drugs market was valued at $2.93 Bn in 2022 and is estimated to expand at a CAGR of 7.16% from 2022-30 and will reach $5.09 Bn in 2030. One of the main reasons propelling the growth of this market is the introduction of newer technologies and drugs, and the aging population. The market is segmented by drug classes and by Distribution Channels. Some key players in this market are Biogen, Eisai Co., Eli Lilly and Company, Johnson and Johnson, H. Lundbeck A/S, F. Hoffmann-La Roche AG, Merck & Co., Novartis AG, and Pfizer among others.
Buy Now

Europe Alzheimer’s Disease Drugs Market Executive Summary
Europe's Alzheimer’s Disease Drugs market was valued at $2.93 Bn in 2022 and is estimated to expand at a compound annual growth rate (CAGR) of 7.16% from 2022 to 2030 and will reach $5.09 Bn in 2030. Alzheimer's disease is a type of dementia that causes problems with memory, thinking, and behavior. The symptoms gradually become severe enough to interfere with daily activities. Alzheimer's disease is the most prevalent form of dementia, which is defined as memory loss and other cognitive deficits that are severe enough to interfere with everyday life. Alzheimer's disease is responsible for 60-80% of dementia cases.
According to Alzheimer’s Europe, the number of persons living with dementia in the European Union (EU27) is projected to be 7 Mn with 9.7 Mn living in European nations represented by AE members. When compared to previous projections, this is a significant decrease from 8.7 Mn for the EU27 and 10.4 Mn for the entire European region. Due to the aging of European populations, the number of persons with dementia in Europe is anticipated to climb to 14 Mn by 2040. In recent years, the European Union, including the European Commission, the Council of the European Union, and the European Parliament, has taken several initiatives in support of dementia. These acts range from political pronouncements and conferences that have raised the visibility of dementia at the European level to financed programs and tools that have supported dementia policy, practice, and research.
Market Dynamics
Market Growth Drivers
Europe has one of the world's largest aging populations, which is another key factor driving growth in the Alzheimer's disease drugs market. As people age, they are at a higher risk of developing the disease, and as the population continues to age, the demand for drugs to treat Alzheimer's disease is likely to continue to increase. According to Eurostat data, more than one-fifth of the EU population was 65 or older in 2020, representing a 3% rise from 2010. Life expectancy in Europe is also rising, rising from an average of 77.7 years for those born in 2002 to 81.3 years for those born in 2019. There have been significant advancements in research and development of new drugs for Alzheimer's disease. This has led to the development of new drugs that are more effective and have fewer side effects than older drugs, which has increased the demand for these new treatments. Governments in Europe are providing support for the development of drugs to treat Alzheimer's disease. This includes funding for research and development, as well as regulatory support to help get new drugs approved for use in the market. The prevalence of Alzheimer's disease in Europe is on the rise, with an increasing number of people being diagnosed with the disease each year. This is one of the key drivers of growth in the market, as it has led to an increased demand for drugs to treat the condition.
Market Restraints
Despite the fact that various medications are available to treat Alzheimer's disease, their efficacy is limited and they sometimes only provide brief relief of symptoms. This can make meeting the requirements of patients and their families difficult, as well as limiting the market growth. There are currently no disease-modifying treatments for Alzheimer's disease, which may limit the efficacy of existing medications. Disease-modifying medicines have the ability to decrease disease progression, and their absence constitutes a huge unmet market need. The regulatory standards for new Alzheimer's disease treatments are strict, making it difficult for companies to get their drugs approved. This can cause delays in the release of new treatments and increase development expenses.
Competitive Landscape
Key Players
- Eisai Co.
- Biogen
- Eli Lilly and Company
- H. Lundbeck A/S
- Johnson and Johnson
- F. Hoffmann-La Roche AG
- Novartis AG
- Pfizer
Recent Updates
The greatest contribution of the European Alzheimer's Alliance (EAA) has been building up the work of the European Dementia Ethics Network, as well as the close follow-up and monitoring of the development and implementation of national strategies to address memory-disabling diseases in various member states.
Healthcare Policies and Regulatory Landscape
Jan 2023: Mental Health Initiative
Dementia is a policy issue that affects several areas, including health, research, disability rights, and elderly people. Within healthcare systems, the illness is frequently treated by many medical disciplines, including mental health and psychiatry, neurology, and gerontology.
Dementia falls under mental health in the WHO and WHO Europe administrative frameworks, and dementia is especially addressed by the latter through the European Framework for Action on Mental Health (EFAMH) and the related Mental Health Coalition, in which Alzheimer Europe participates. There is a dedicated workstream within EFAMH focusing on the mental health of older people, which focuses on dementia and other causes of poor mental health in older people (e.g. social isolation, stigma, etc.)
Nov 2007: European Alzheimers Alliance
Members of the European Parliament (MEPs) convened in Paris and formally launched the European Alzheimers Alliance, a collaboration between Alzheimer Europe and its member organizations to make dementia a European public health priority (EAA). The international and cross-party Alliance gathered Parliamentarians to support European persons suffering from Alzheimer's disease or another form of dementia, as well as informal or family carers.
Reimbursement Scenario
The reimbursement scenario for Alzheimer's drugs in Europe can vary depending on the country and the specific drug in question. Generally, in Europe, the reimbursement of drugs is the responsibility of individual countries, which may have different policies and procedures for reimbursement.
In many European countries, Alzheimer's drugs are reimbursed under national health systems, which provide coverage for the cost of drugs to patients who meet certain eligibility criteria. These criteria may include factors such as disease severity, age, and other medical conditions.
In some countries, such as the UK, Alzheimer's drugs are provided free of charge to patients through the National Health Service (NHS), while in other countries, patients may be required to make a co-payment for the drugs.
1. Executive Summary
1.1 Disease Overview
1.2 Global Scenario
1.3 Country Overview
1.4 Healthcare Scenario in Country
1.5 Patient Journey
1.6 Health Insurance Coverage in Country
1.7 Active Pharmaceutical Ingredient (API)
1.8 Recent Developments in the Country
2. Market Size and Forecasting
2.1 Epidemiology of Disease
2.2 Market Size (With Excel & Methodology)
2.3 Market Segmentation (Check all Segments in Segmentation Section)
3. Market Dynamics
3.1 Market Drivers
3.2 Market Restraints
4. Competitive Landscape
4.1 Major Market Share
4.2 Key Company Profile (Check all Companies in the Summary Section)
4.2.1 Company
4.2.1.1 Overview
4.2.1.2 Product Applications and Services
4.2.1.3 Recent Developments
4.2.1.4 Partnerships Ecosystem
4.2.1.5 Financials (Based on Availability)
5. Reimbursement Scenario
5.1 Reimbursement Regulation
5.2 Reimbursement Process for Diagnosis
5.3 Reimbursement Process for Treatment
6. Methodology and Scope
Alzheimer's Disease Drugs Market Segmentation
By Drug Class (Revenue, USD Billion)
- Cholinesterase Inhibitors (Donepezil, Rivastigmine, Galantamine)
- N-Methyl-D-Aspartate (NMDA) Receptor Antagonists (Memantine)
- Combination Drugs
- Others (Lecanemab, Aducanumab)
Drug segmentation is the process of dividing a set of drugs into different categories or classes based on their pharmacological properties, therapeutic uses, and other characteristics. Here, Alzheimer’s Disease Drugs Market is segmented into Cholinesterase Inhibitors, N-Methyl-D-Aspartate (NMDA) Receptor Antagonists, Combination Drugs, and others like (Lecanemab, Aducanumab)
By Distribution Channel (Revenue, USD Billion)
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Route of Administration
- Oral
- Transdermal
- Intravenous
Methodology for Database Creation
Our database offers a comprehensive list of healthcare centers, meticulously curated to provide detailed information on a wide range of specialties and services. It includes top-tier hospitals, clinics, and diagnostic facilities across 30 countries and 24 specialties, ensuring users can find the healthcare services they need.
Additionally, we provide a comprehensive list of Key Opinion Leaders (KOLs) based on your requirements. Our curated list captures various crucial aspects of the KOLs, offering more than just general information. Whether you're looking to boost brand awareness, drive engagement, or launch a new product, our extensive list of KOLs ensures you have the right experts by your side. Covering 30 countries and 36 specialties, our database guarantees access to the best KOLs in the healthcare industry, supporting strategic decisions and enhancing your initiatives.
How Do We Get It?
Our database is created and maintained through a combination of secondary and primary research methodologies.
1. Secondary Research
With many years of experience in the healthcare field, we have our own rich proprietary data from various past projects. This historical data serves as the foundation for our database. Our continuous process of gathering data involves:
- Analyzing historical proprietary data collected from multiple projects.
- Regularly updating our existing data sets with new findings and trends.
- Ensuring data consistency and accuracy through rigorous validation processes.
With extensive experience in the field, we have developed a proprietary GenAI-based technology that is uniquely tailored to our organization. This advanced technology enables us to scan a wide array of relevant information sources across the internet. Our data-gathering process includes:
- Searching through academic conferences, published research, citations, and social media platforms
- Collecting and compiling diverse data to build a comprehensive and detailed database
- Continuously updating our database with new information to ensure its relevance and accuracy
2. Primary Research
To complement and validate our secondary data, we engage in primary research through local tie-ups and partnerships. This process involves:
- Collaborating with local healthcare providers, hospitals, and clinics to gather real-time data.
- Conducting surveys, interviews, and field studies to collect fresh data directly from the source.
- Continuously refreshing our database to ensure that the information remains current and reliable.
- Validating secondary data through cross-referencing with primary data to ensure accuracy and relevance.
Combining Secondary and Primary Research
By integrating both secondary and primary research methodologies, we ensure that our database is comprehensive, accurate, and up-to-date. The combined process involves:
- Merging historical data from secondary research with real-time data from primary research.
- Conducting thorough data validation and cleansing to remove inconsistencies and errors.
- Organizing data into a structured format that is easily accessible and usable for various applications.
- Continuously monitoring and updating the database to reflect the latest developments and trends in the healthcare field.
Through this meticulous process, we create a final database tailored to each region and domain within the healthcare industry. This approach ensures that our clients receive reliable and relevant data, empowering them to make informed decisions and drive innovation in their respective fields.
To request a free sample copy of this report, please complete the form below.
We value your inquiry and offer free customization with every report to fulfil your exact research needs.








































